March 8, 2024
By Sade Sobande
The MHRA webinar (MedTech Regulatory Reform Webinar, March 5) gave insight into the UK government’s plans for implementing the future regulation of medical devices (including IVDs).
Software as a medical device was specifically called out. Concerning device classification, MHRA confirmed, as we have reported before, that they will adopt the risk categorization in IMDRF: "Software as a Medical Device": Possible Framework for Risk Categorization and Corresponding Considerations. This furthers the UK regulator’s goal for international alignment. It should be noted, that the IMDRF framework has only been adopted for Software as Medical Devices (SaMD) that are general medical devices, not IVDs.
EU Alignment with IMDRF software
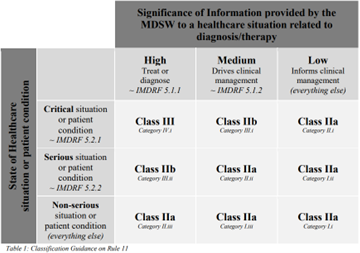
The EU Medical Devices Regulation (2017/745, MDR) also aligns with the IMDRF in general. However, while it remains to be seen exactly how the UK regulation will be written and interpreted, MHRA specified that they will align with the IMDRF risk categorization framework, and not the EU MDR is particularly interesting. It should be clarified that there is no huge divergence between the EU MDR medical device software classification as per Rule 11 and the IMDRF risk categorization. In fact, Rule 11 was introduced to closely mirror the IMDRF framework. There is, consequently, a useful table in Annex III of MDCG 2019-11.

The exclusion of class I devices from this table, while explicitly stated, is also the crux of potential differences in the classification of SaMD between the UK and EU markets. Note the EU reference to software products as Medical Device Software (MDSW).
MHRA future legislation and SaMD
In the webinar, the MHRA states full alignment with the IMDRF classification as follows.
- IMDRF Category I = UK MDR Risk Class I
- IMDRF Category II = UK MDR Risk Class IIa
- IMDRF Category III = UK MDR Risk Class IIb
- IMDRF Category IV = UK MDR Risk Class III
Comparison of SaMD in the UK and MDSW in the EU
How the discussion about future regulations has been advanced means that a manufacturer could potentially have SaMD that is regulated as Class I in the UK, but Class IIa in the EU. The wording of EU MDR, Annex VIII, Rule 11 which has led a significant number of MDSW to be up-classified also seems to allocate a significant number of medical device software to higher classes:
Software intended to provide information that is used to make decisions with diagnosis or therapeutic purposes is classified as Class IIa, except if such decisions have an impact that may cause:
- death or an irreversible deterioration of a person’s state of health, in which case it is in Class III; or
- a serious deterioration of a person’s state of health or a surgical intervention, in which case it is classified as Class IIb.
Software intended to monitor physiological processes is classified as Class IIa, except if it is intended for monitoring of vital physiological parameters, where the nature of variations of those parameters is such that it could result in immediate danger to the patient, in which case it is classified as Class IIb.
All other software is classified as Class I.
The wording of Rule 11 is such that a manufacturer of an MDSW device would de facto start with an initial Class IIa classification.
Concluding remarks
While the MDCG guidance (MDCG 2019-11) in some ways provides a more pragmatic interpretation of Rule 11, the illustration in Annex III substantiated by the explanatory note that: “The IMDRF risk category II and IMDRF risk category I products are classified as MDR Class IIa as per Rule 11.”, may result in manufacturers of SaMD products only being able to market their UK Class I devices as Class IIa in the EU.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.







