June 24, 2020
In the complex world of medical device regulation, it can be difficult to keep things straight when it comes to product development. Different methods are needed to demonstrate that your production-ready medical device is safe and effective and meets its intended users’ needs. These methods generally fall under the umbrella of design verification and validation (V&V). However, the terms “design verification” and “design validation” are so often lumped together that it can be difficult to distinguish between the two activities. To add to the confusion, US FDA defines a separate validation test, referred to as human factors validation, that seemingly exists in a category of its own.
Definitions and perspectives
This post seeks to clarify the differences between each phase of the V&V process and to provide an example of how design verification, design validation, and human factors validation would be used to demonstrate that a specific user need is met. Before we dive in, it’s important to point out that different medical device manufacturers might think differently about what constitutes design verification, design validation, and human factors validation. This post represents just one perspective, while other perspectives still may be completely valid and meet the needs of regulatory requirements.
First, let’s start off with some definitions. FDA provides the following language on verification and validation in the code of federal regulation (CFR) 21 part 820.30:
“Design verification shall confirm that the design output meets the design input requirements” and “Design validation shall ensure that devices conform to defined user needs and intended uses and shall include testing of production units under actual or simulated use conditions” (see Code of Federal Regulations Title 21).
But what exactly does this mean? A lot of people like to think of verification as asking, “Did you design your device right?” and validation as “Did you design the right device?” In other words, verification provides evidence that your device meets its product requirements (i.e., design inputs). This is largely accomplished through benchtop testing, but it can also be done through certain analyses such as a drawing inspection or failure modes effect analysis. Validation, on the other hand, confirms that your product meets its user needs. This is done by performing whole system tests with production or production-equivalent units. The relationship between verification and validation is depicted by FDA’s graphic below:
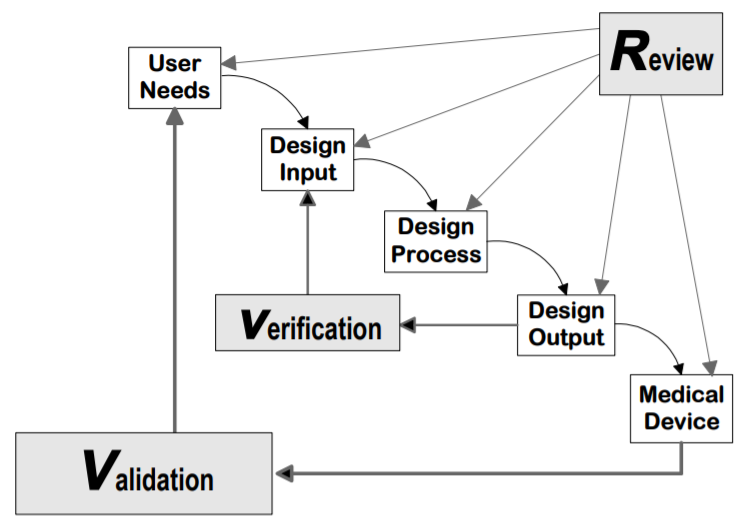
Notably, human factors validation is considered part of design validation (see FDA's final guidance, Applying Human Factors and Usability Engineering to Medical Devices, section 3.7). So how does human factors validation differ from design verification and validation? While design validation’s overall goal is to confirm that a device’s user needs are met, human factors validation specifically evaluates the device’s user interface to confirm that the device can be used safely and effectively by the intended users in the intended use environment.
The table below provides a summary of design verification, design validation, and human factors validation.
|
|
Design verification |
Design validation |
Human factors validation |
|---|---|---|---|
|
Summary |
Benchtop testing, analysis, and/or inspection to confirm design outputs meet design input requirements |
Simulated/actual use testing (including human factors validation and clinical testing) to confirm user needs are met |
Evaluation of user interface to confirm intended users can interact with device safely and effectively |
|
Test units |
Production units, production-equivalent units, or device subsystems |
Production units or production-equivalent units
|
Production units or production-equivalent units, including all instructional materials, training, and labeling |
|
Timeline |
During development cycle to end of development cycle |
End of development cycle |
End of development cycle |
Verification and validation in real design processes
Now, let’s look at a real-world example of when you would conduct design verification, design validation, or human factors validation.
Suppose you are developing a medical device for use by paramedics with the following user need: “I need to use the device in an ambulatory setting.” This user need would be translated into several product requirements (or design inputs), likely related to the device’s size, weight, and battery life, to verify that the device was suitable for an ambulatory setting. These product requirements should be written so they can be objectively assessed. For example, there could be a product requirement stated as: “The battery shall last at least 12 hours with the device operating at maximum power consumption.” The product requirement provides a more concrete element to test or verify. For example, you might verify this requirement by running production-equivalent devices in a lab environment from maximum charge until battery depletion, enabling you to verify that the battery lasts at least 12 hours – in other words, that you designed the product right.
After the device is fully designed, you would need to validate that the user need is actually met. In other words, can paramedics effectively use the device in an ambulatory setting? This could be done by testing the device in the field, noting that device use in an actual or simulated use environment can highlight issues that might get overlooked during the development cycle or in lab-based testing. For example, perhaps an element of the use environment, such as temperature, affects the battery life. Or, maybe the product requirement for a 12-hour battery life is actually insufficient, and healthcare professionals need the device battery to last 14 hours. Testing your device in the actual or simulated environment is imperative to ensuring your device works as intended – in other words, that you designed the right product.
So, where does human factors validation come into play? Let’s imagine that a use-related risk analysis reveals a critical task related to charging the device, meaning that if a user fails to charge the device properly, the battery could fail out in the field when it is most needed, leading to patient harm. Although the battery might have passed verification and design validation, human factors validation might reveal that users don’t realize the device needs to be charged daily, don’t know how to perform the charging process, or don’t properly interpret the battery life indicator. So, even if the battery is designed to last an entire shift, if it’s susceptible to misuse, it cannot – in essence – be used safely and effectively.
Hopefully this example provides some clarity around design verification, design validation, and human factors validation. Even if you define these activities slightly differently, the most important thing to remember is that you should take a holistic perspective to help your device provide a safe and effective (and satisfying) experience in the hands of intended users.
Regan Via is Human Factors Specialist at Emergo by UL’s Human Factors Research & Design division.
Learn more about HFE and usability testing for medical devices:
- HFE user research support for medical devices and IVDs
- Human factors design and prototype development support
- Medical device and product evaluation and usability testing
- Webinar: Human factors engineering for medical devices
- Webinar: Examining methods for identifying potential use errors
Request more information from our specialist
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.







