October 7, 2025
Tom Ingless and Annette van Raamsdonk
Last year, we shared an update on the gradual rollout of the European Database on Medical Devices (EUDAMED). While deadlines have shifted again, the European Commission (EC) is preparing stakeholders for registering devices in the Unique Device Identification (UDI)/Device Module.
On Sept. 30 and Oct. 1, Emergo by UL participated in the EC’s EUDAMED onboarding training for the UDI/Device module.
Current timeline
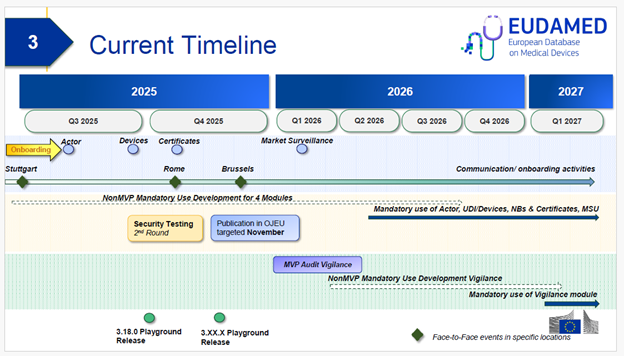
The timeline for EUDAMED’s implementation has shifted, with the first notice on the readiness of the Actor, UDI/Device. Notified Bodies (NB) and Certificates, and Market Surveillance modules are expected to be published in the Official Journal of the European Union (OJEU) in mid-November 2025.
Once the notice in the OJEU is published, the obligations and requirements for device registration in EUDAMED’s UDI/Device Module become mandatory after 6 months. With the new timeline in mind, it is expected that this will now be in mid-May 2026. However, the timelines for performing said registrations vary depending on when the first sales unit of the device was placed onto the EU market:
- When the first sales unit of a new Regulation device (compliant with the MDR or IVDR) is to be placed onto the EU market on or after the mandatory use date of the UDI/Device Module (mid-May 2026), the registration must be performed before the device is allowed to be placed onto the market. (NB: A “new Regulation device” in this context refers to an entirely new device, not one belonging to a generic device group which is already on the market.)
- When the first sales unit of a legacy device (compliant with the MDD, AIMDD, or IVDD) with an appropriate transition extension or an existing Regulation device was placed onto the market before the mandatory use date of the UDI/Device Module (mid-May 2026), the device will have a 12 month transition period to be registered which starts from the publication date of the notice in the OJEU, i.e., these devices must be registered in the UDI/Device Module by mid-November 2026.
Legacy and Regulation devices which are no longer being placed on the EU market before the mandatory use date of the UDI/Device Module (mid-May 2026) but of which sales units are still circulating in the distribution chain after the mandatory use date do not need to be registered, unless a post-market surveillance or vigilance-related issue occurs which needs to be reported in EUDAMED after mandatory use of the Vigilance Module begins. (NB: The Vigilance Module will not be a part of the initial notice of readiness; its use is not expected to become mandatory before Q2 2027).
Summary of the EUDAMED onboarding training
The EC provided stakeholders with onboarding training so that they could train their members. Emergo by UL, along with other European Authorized Representatives, attended the two-day training and is preparing a webinar on EUDAMED registrations. We will touch on some of the topics discussed.
Types of device identifiers and their hierarchy relationships
EUDAMED uses several types of device identifiers to manage and track devices:
- Basic Unique Device Identification–Device Identifier (UDI-DI): The core identifier grouping devices with the same intended purpose and design. It acts as an umbrella for multiple UDI-DIs and is mandatory for regulated devices.
- UDI-DI: A unique identifier for a specific device model. It appears on device labeling and is used for traceability.
- Package UDI-DI: Identifies packaging configurations and quantities at each package level.
- Master UDI-DI: This is used for special device types (e.g., contact lenses) to simplify registration. It replaces multiple UDI-DIs with a single identifier for grouped products.
- The identifier hierarchy in EUDAMED is structured as follows:
- One Basic UDI-DI can be linked to multiple UDI-DIs
- Each UDI-DI can have associated Package UDI-DIs
- Registration of a Basic UDI-DI must include at least one UDI-DI
- Additional UDI-DIs can be added later through device management
Device registrations for legacy devices
Legacy devices refer to medical devices, in vitro diagnostic devices, or active implantable devices that were placed on the EU market under previous directives (MDD, AIMDD, IVDD) and continue to be marketed during the transition to MDR/IVDR. These devices are not yet certified under the new regulations but remain available under transitional provisions unless significant changes in design or intended purpose occur. These devices do not require registration in EUDAMED, unless the PMS Vigilance module is live and a vigilance event occurs.
The registration of “Regulation Devices.” Regulation devices refer to medical devices and in vitro diagnostic medical devices that are placed on the EU market under the MDR or IVDR.
What data do you need to keep ready when registering these devices?
• Basic UDI-DI and UDI-DI codes
• European Medical Device Nomenclature (EMDN) codes and trade names
• Device characteristics (e.g., size, sterilization, warnings)
• Market distribution details
• Clinical investigation information (if applicable)
• Substances (e.g., carcinogenic, mutagenic or reprotoxic (CMR), endocrine disruptors) if present- Linking legacy devices and regulation devices
- Bulk Upload Functionality
- Managing versions
Concluding remarks
With this two-day training, the EC prepares stakeholders for the mandatory use of EUDAMED. While the targeted evaluation of the Regulations is ongoing, the implementation and rollout of EUDAMED are as well. Emergo by UL is preparing an EUDAMED webinar to help manufacturers, system and procedure pack producers, and importers prepare so they know what to expect when the UDI/Device Module of EUDAMED becomes mandatory.
Emergo by UL takes part in several European Commission Medical Device Coordination Groups (MDCG) and will keep you updated on any developments. For further information, make sure you are signed up for our newsletter.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.