June 13, 2021
Over the last decade, pharmaceutical and medical device manufacturers have marketed numerous combination products that include lyophilized (i.e., freeze-dried) drugs. (We can define a combination product as being composed of any combination of a drug and a device; a biological product and a device; a drug and a biological product; or a drug, a device, and a biological product.) Along with the growing increase of marketed combination products, we discern an emerging pattern for how the pharmaceutical industry reconstitutes lyophilized drugs. In this blog post we want to shed light on a few of these innovative reconstitution technologies that might soon be adopted by more manufacturers.
Understanding lyophilization and reconstitution of pharmaceutical products
But first, what is lyophilization? Lyophilization has become a commonly used preservation technique for biological products or drugs in the pharmaceutical industry. The reason for this is that the process of lyophilization does not require high temperatures that could cause the denaturing of proteins and other critical biological components. Lyophilization allows for safe storage without placing the product’s or drug’s effectiveness at risk.
Now you might be asking yourself, how do reconstitution devices fit into all of this? After a lyophilized pharmaceutical product is distributed to the market, it cannot be injected into a patient until it has been reconstituted. The process of reconstitution consists of mixing a diluent such as sterile water or normal saline solution to liquefy a dehydrated substance (e.g., therapeutic medication). To this day, healthcare professionals are trained in traditional multi-step reconstitution procedures, which in some cases can take up to 30 minutes. The process requires interaction with a reconstitution kit, typically made up of several pieces of equipment, including but not limited to a medication vial, a diluent vial, a syringe, a reconstitution needle, and an injection needle. A recent example of this is Pfizer-BioNTech’s COVID-19 vaccine, which requires traditional vial and syringe reconstitution before administration.
When manufacturers need to decide on which reconstitution device or system to choose, essential factors that come into play include budget, timeline, and the targeted product or drug. That said, a central concern will always be finding the device or system that can be used most safely and effectively by its intended users in their intended use environments. The solution to this search will depend on the characteristics of these users and environments. Though it might seem like the emerging reconstitution technologies vary greatly, the innovations all serve as engineered solutions for a common set of goals: reducing the time for reconstitution, the number of required components, and the occurrence of errors, while progressively moving the administration of drugs into the hands of laypeople.
Types of advanced reconstitution devices for lyophilized drugs
In recognition of these needs, manufacturers have innovated greatly in developing advanced reconstitution devices such as dual-chamber pre-filled syringes, vial adapters, automated reconstitution systems, and others.
- Dual-chamber pre-filled syringes: This syringe consists of two chambers: one with a lyophilized product (e.g., powdered medication) and one with a diluent (e.g., sterile water). The two chambers are separated by a rubber stopper, and the two substances (i.e., powder and diluent) remain unmixed until administration. To reconstitute the medication, the user presses down on the syringe’s plunger, causing the diluent to flow into the adjacent chamber and mix with the lyophilized product.
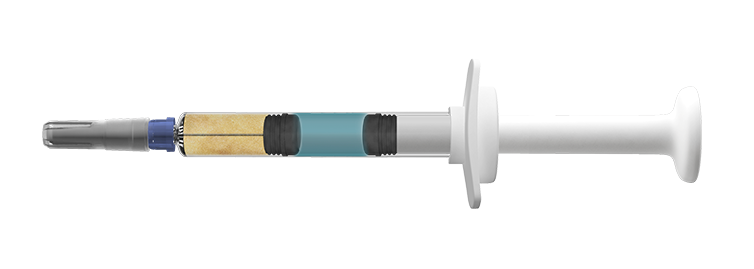
Figure 1. Credence Companion Dual Chamber Reconstitution Safety Syringe. (Source)
- Vial adapters: Generally, one end of a vial adapter consists of a standard female Luer lock to fit standard Luer taper syringes. The opposite end consists of a shielded spike to fit the outer diameter of a vial stopper. The tip of a syringe is placed in the Luer lock connector end, and diluent is then withdrawn from the vial. The vial adapter and syringe are separated from the vial once the right amount of diluent has been withdrawn. The spike tip of the vial adapter is then pushed into the rubber stopper of the medication vial. The diluent is expelled into the drug powder vial to dissolve the powder. The reconstituted medication can then be withdrawn into the syringe for administration.

Figure 2. West Pharma Vial Adapters. (Source)
- Automated reconstitution systems: The user places a vial with lyophilized product in one receptable of the system and a vial with the corresponding diluent in the remaining receptacle. The user then typically presses a single button to automatically reconstitute the medication.

Figure 3. Enable Injections Automatic Reconstitution Transfer System. (Source)
Errors related to needle-stick injury, bent needles, or broken needles are all known problems in reconstitution systems. The same applies to the number of steps needed to reconstitute the medication. Both of these are areas of focus in developing innovative reconstitution technologies. Notably, many of these technologies have a common design characteristic: needleless reconstitution. They also typically require fewer steps to reconstitute medication, leading to fewer potential use errors.
Conclusion
State-of-the-art reconstitution technologies are developing fast with the goal of reducing the occurrence of use-related problems. Ideally, the technologies should not require more than two steps to reconstitute a lyophilized product or drug. They should also reduce the exposure of needles as much as reasonably possible. This does not indicate that the reconstitution kits currently on the market are highly problematic, but there is a clear need for manufacturers to make progress towards enhanced safety and effectiveness when it comes to drug and vaccine reconstitution.
Frauke Schuurkamp is Managing Human Factors Specialist and Michael Orduz is Human Factors Associate at Emergo by UL’s Human Factors Research & Design (HFR&D) division.
Learn more about HFE and user-centered design for healthcare products:
- HFE user research support for medical devices, IVDs, and combination products
- Medical device design and prototyping support
- Medical device and combination product evaluation
- Webinar: Latest FDA expectations for validation testing of combination products
Request information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.