July 18, 2023
On March 20th, 2023, we informed you that Regulation (EU) 2023/607 was adopted in Europe. The new regulation allows legacy medical devices that fulfill the additional conditions of Article 120 (as amended) to remain in the market till 2027/2028, depending on the class of the devices according to the Medical Devices Regulation (EU) 2017/745 (MDR).
We also informed you about the Q&A on practical aspects related to the implementation of Regulation (EU) 2023/607, which was published a few weeks after Regulation (EU) 2023/607. Although many questions were answered in the initial Q&A document, Emergo Europe as well as other EU Authorized Representatives experienced an influx of questions coming in from manufacturers.
Also, the “self-declaration,” which needs to be drawn up by the manufacturer to declare that the conditions for the extension are fulfilled and state the end date of the transition period, was not published yet.
Revised Q&A on Regulation (EU) 2023/607
On July 18th 2023, the revised Q&A on practical aspects related to the implementation of Regulation (EU) 2023/607, was published. Stakeholders including the European Association of Authorized Representatives (EAAR) have been contributing to this updated document during the Medical Device Coordination Group (MDCG) workshop in June.
So what has changed?
Two questions have been revised; six questions have been added.
The most important topics that have been addressed in the revised Q&A:
- Clarity on when the transitional provisions apply (Q 6.1 & 6.2)
- The manufacturer’s self-declaration is added to the Q&A document in section 7, and can also be found on the European Commission website
- Q 10.1. is added: “Can another manufacturer lodge the application for conformity assessment of the legacy device?”
- Clarity on when Unique Device Identifier (UDI) requirements apply
- Added: requirements that need to be met by the manufacturer when switching to another Notified Body
Additionally, Notified Bodies call out not to wait till 2024 for submitting applications. The application and signed Notified Body agreement is needed in order to be eligible for the extension of the CE certificate(s). Notified Bodies fear that because limited documentation needs to be provided, the chances a manufacturer doesn’t pass the audit in time are a realistic situation.
So, don’t wait till the last moment with your application, as you might run the risk of not being transferred to the MDR in time, and losing European medical device market access.
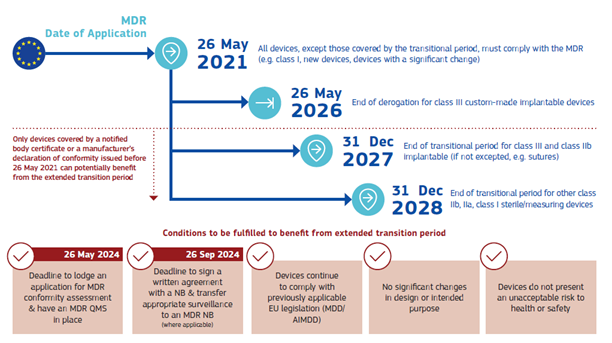
Last but not least, also an updated “Factsheet for authorities in non-EU/EEA countries” is available to avoid miscommunication on the extension of certificates issued under the MDD/AIMDD. The document contains a helpful flow chart of the new deadlines:

P. 4 Factsheet for authorities in non-EU/EEA countries
Emergo by UL consultants can support manufacturers manage their Notified Body relationships, MDR transition efforts and Regulation (EU) 2023/607 qualifications.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.







