Thank You for Registering for our HFR&D Webinar
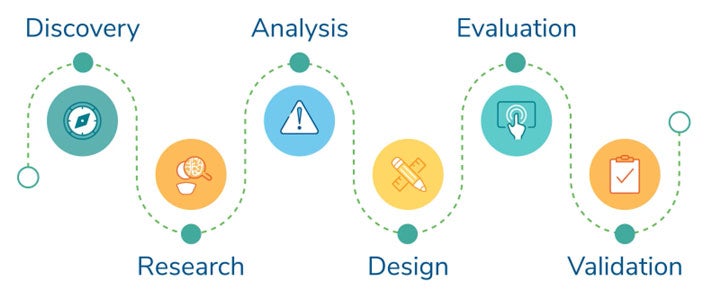
We look forward to seeing you! In the meantime, discover how our Human Factors Research & Design team of experts can empower the development of products with a safer, more effective and superior user experience.

Related content






