April 19, 2023
By Michael Rothstein
What is a sharps injury prevention feature?
A sharps injury prevention feature is a component of some injection devices intended to prevent needlestick injuries. Some devices include active sharps injury prevention features, which users must manually activate to cover a needle after performing an injection (e.g., snap-down needle safety covers); other devices include passive sharps injury prevention features, which automatically cover a needle after performing an injection (e.g., autoinjector tips that lock after injecting).
What is sharps injury prevention testing?
Sharps injury prevention tests are simulated clinical use tests intended to validate a sharps injury prevention feature’s infection prevention efficacy by verifying that needlestick injuries are statistically unlikely to occur. The US Food and Drug Administration considers a 500-injection test with zero failures to be sufficient to detect grossly defective devices at the 1% level. In a sharps injury prevention test, a failure is defined as 1) a needlestick injury, or 2) an instance in which the sharps injury prevention feature does not activate after a user injects using proper injection technique.
Even if a device with a sharps injury prevention feature will be used by laypeople, FDA recommends including only healthcare professionals in the sharps injury prevention test to avoid learning curve artifacts. To minimize bias, FDA recommends that the test be conducted in at least two cities with at least eight total evaluators. To limit physical and mental fatigue-based errors, Emergo by UL recommends including more than the minimum eight evaluators. To determine the number of evaluators, consider the time and physical strength required to perform each injection. For example:
-
A safety lancet is quick and requires little force; 10 evaluators who perform 50 lances should be sufficient.
-
A pre-filled syringe requires more time and force to press the plunger down to administer the injection; we would recommend including 20 evaluators to each perform 25 injections.
When do you need to conduct a sharps injury prevention test?
FDA recommends that sharps injury prevention tests be conducted on novel sharps injury prevention features. If a sharps injury prevention feature is currently marketed as part of another device, identifying that device in your FDA submission is sufficient in lieu of a sharps injury prevention test.
Key factors when designing a sharps injury prevention test
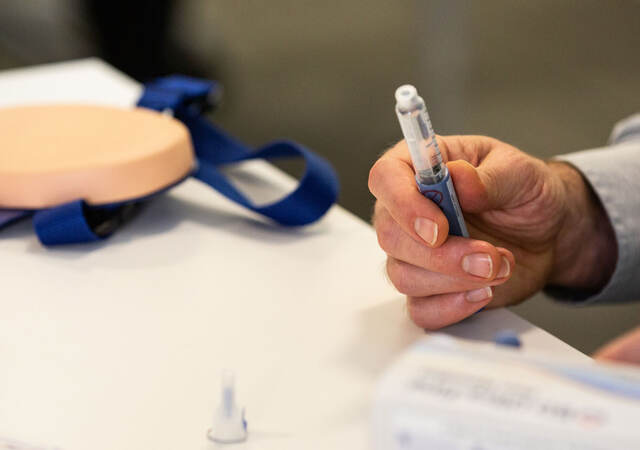
Environment design. Sharps injury prevention tests are simulated clinical use tests, meaning evaluators should inject into injection cushions rather than an actual patient. However, sharps injury prevention tests should consider performance-shaping environmental factors. For example, if an evaluator would wear gloves when using this device in their workplace, they should wear gloves during the sharps injury prevention test. If they would perform injections with one hand or with wet hands in their workplace, they should perform their injections during the sharps injury prevention test with one hand or with wet hands.
Evaluator training. The test moderator should train the evaluator to use the injection device and verify that the evaluator understands proper injection technique. Training might consist of an instructional video and/or a detailed review of the device’s instructions for use. The test moderator should also demonstrate proper injection technique and ask the evaluator to perform a return demonstration. If the evaluator demonstrates using improper technique, they should perform additional demonstration injections until their technique is correct.
Report forms. Sharps injury prevention test results should be tracked on a report form. Report forms should include methods to collect the following types of data:
-
Test session logistical information (e.g., date/time, location, test moderator)
-
Evaluator background information (e.g., age, sex, handedness, job title, experience with sharps injury prevention devices)
-
Success/failure of each of the injections
-
Deviations from proper injection technique
-
Evaluators’ subjective feedback about the sharps injury prevention feature
For more information on sharps injury prevention features and sharps injury prevention tests, see FDA’s Guidance for Industry and FDA Staff; Medical Devices with Sharps Injury Prevention Features. Sharps injury prevention tests are detailed in Section 10, “Simulated Clinical Use Testing.”
Michael Rothstein is Managing Human Factors Specialist at Emergo by UL’s Human Factors Research & Design division.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.