In-depth medical device analysis processes
Our human factors specialists conduct comprehensive analyses to design safer, more effective and user-friendly medical devices according to regulatory expectations in terms of applying human factors engineering (HFE) in product design and development. Our human factors toolkit contains many types of analyses.
Use-Related Risk Analysis (URRA)
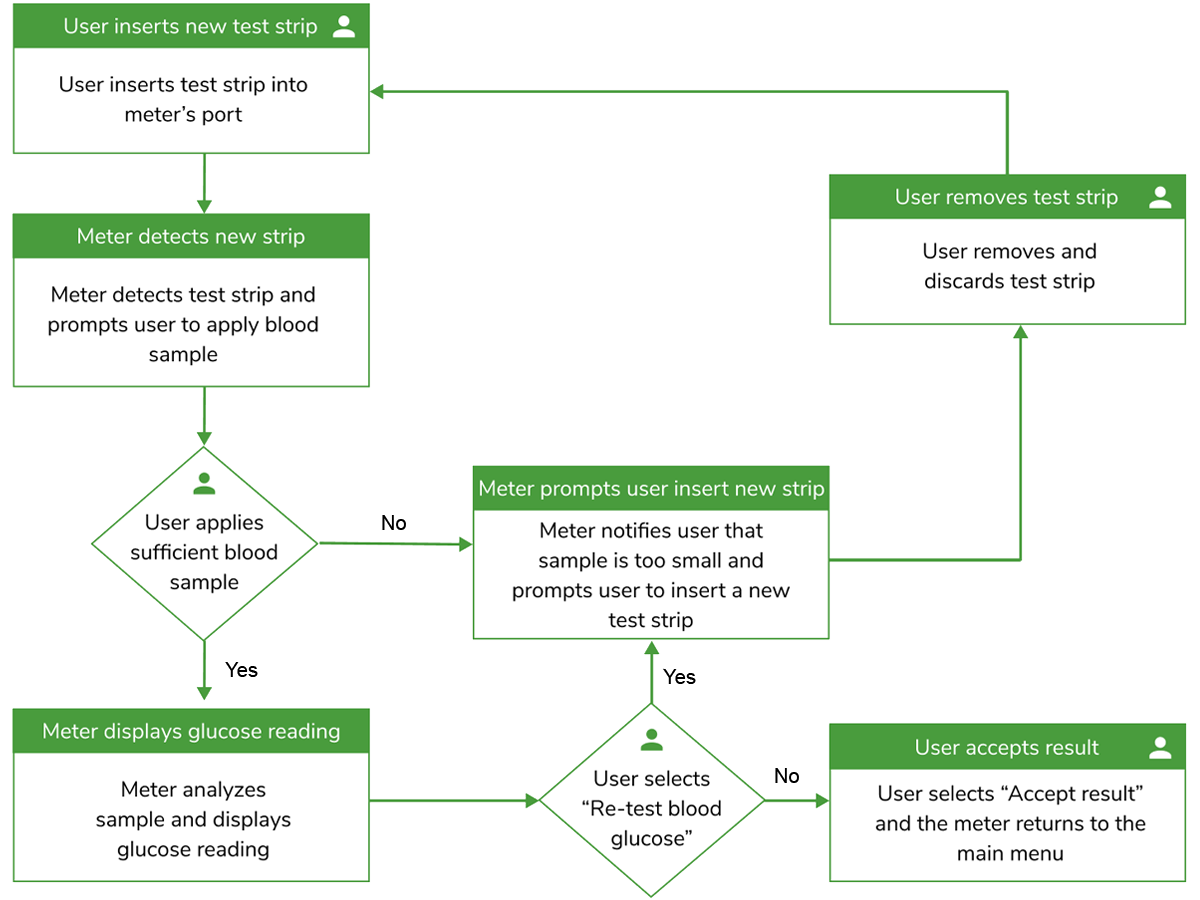
One of the most common types of analyses we lead for our customers is a comprehensive, use-related risk analysis, which is central to scoping HFE work for any medical device or combination product. The URRA is needed to help teams create products that can be more safely and effectively used and to meet the expectations of the U.S. Food and Drug Administration (FDA) and other regulators. Our technical approach to use-related risk analysis calls for the identification of all possible use errors associated with anticipated use scenarios.
Use-related medical device analysis activities
Task analysis
Identifying potential use errors can start with a task analysis. We can analyze a product’s user interface quite easily, based on a product’s preliminary, refined or final form, by doing a detailed walkthrough of product use and cataloging every use step and sequence. Then, for each step, we consider discrete user Perceptions, cognitive steps, and actions (PCA analysis). This activity uncovers opportunities to increase user interaction efficiency and provides high visibility on use errors that might be reduced or eliminated.
Hazard analysis
Our hazard analyses complement our task and use-related risk analyses. Instead of looking at a task flow and considering what use errors could lead to harm, a hazard analysis assumes harm and then considers what errors could cause it. Therefore, we might start our analysis by assuming harms of the following forms: burns (thermal and chemical), electrical shock, biological contamination, i.e., infection, radiation exposure, and various types of physical and even emotional trauma. Then, we analyze what use errors and other factors could lead to the specified harm.
URRA (Use-FMEA) table
Often starting with a task analysis, we analyze each use scenario step by step and identify potential use errors, hazards, harms and associated severities, ultimately enabling the identification of critical tasks and linkage to key risk mitigations. While risk estimation is determined by both the severity of the potential harm and the likelihood of the error and potential resulting harm, it is the severity level of potential harm that most determines the nature of use-related risk mitigations as well as the scope of HFE activities, including HF validation testing.
Typically, using a tabular format, we strive to document the ways that people could make a mistake while interacting with a product in the presence of various performance-shaping factors, including environmental and user-related characteristics. Depending on your organization’s requirements, the URRA table can be part of a more comprehensive risk management analysis, such as a Use-FMEA.
Known-problems analysis (KPA)
A KPA helps identify how people have made mistakes or otherwise struggled using products similar to the one being developed. Regulatory guidance and recognized standards specifically call upon manufacturers to perform this “identification of known use-related problems,” a structured initiative to learn from the past, as a key input to the URRA for the product in development. The analysis’ scope is focused on predicate and/or marketed products with similar user interfaces, user interactions and/or uses.
Analysis inputs include a review of information, i.e., reports, found in adverse event databases, e.g., Manufacturer and User Facility Device Experience (MAUDE), available data in customer complaints systems and insights from product trainers and users. The end product of our analysis is usually an extensive list of problems articulated in terms of a given use error. Then, alongside each error, we cite risk mitigations implemented into the device to guard against that use error — or identify the need to implement a mitigation that can then be converted into a user interface requirement for further development.
Adverse event analysis
Our analyses involve studying adverse events that have occurred during medical device use to identify potential user interaction root causes. We review the event from the perspective of the human factors suitability of the product’s user interface and consider other performance-shaping factors. We then develop a hypothesis of how a design flaw induced the adverse event, or we conclude that the user interface was not a contributing cause.
Our human factors specialists have been called upon to study adverse events and determine their most likely root causes. Some cases:
- Determine how the user interface of a surgical suction system might have induced members of surgical teams to apply high-pressure suction to a passive chest drain, thereby causing fatal lung injuries.
- Determine how the user interface of a hemodialysis machine induced dialysis nurses and technicians to overlook that the machine was removing too much fluid from patients, thereby causing hypovolemia (lack of fluid in the body), leading to death or injury.
- Determine how the user interface of an automated external defibrillator induced users to conduct battery tests in a way that inadvertently left the device discharged and unable to function when it was needed to save persons experiencing life-threatening arrhythmias.
Anthropometric analysis
Our specialists are well-versed in the long-established science of anthropometric analysis. We draw upon extensive data on the size, shape, strength, range of motion and other physical characteristics of humans to perform geometric studies (in 2D and 3D). These analyses help us determine how well a product is matched to the intended users’ physical traits.
Examples of anthropometric analysis include:
- Collecting the anthropometric and ergonomic data of combination device users and recommendations for appropriate design features.
- How much force a fifth-percentile female can apply to a lever to open a malfunctioning, semi-automated drug delivery machine in an emergency.
- The proper dimensions of a surgical instrument so that it can be held comfortably and actuated properly by individuals with very large hands as well as those with very small hands.
- The proper size and placement of components comprising a sit-stand workstation that is operated by surgeons performing robot-assisted surgery.
HFE market analyses for medical devices
Gap analysis
Our customers routinely call upon us to review their completed HFE activities and associated documentation against one or more sets of HFE expectations, e.g., FDA guidance or IEC 62366, and to identify “gaps” that should be filled to help ensure a complete design history and/or usability engineering file. Our specialists can lead the effort to filling any gaps, performing and/or documenting the missing HFE activities, or support our customers as they lead these activities.
Threshold and comparative analyses
We systematically identify differences between a reference product and proposed generic or a marketed predicate and new device in development. We might perform these analyses to help a customer comply with regulators’ expectations, e.g., FDA’s guidance regarding applying HFE to proposed generic products, or to generate data justifying that HFE data for an existing product can apply to — and, therefore, reduce the scope of HFE activities required for — a product in development.
State of the art (SOTA) analysis
The EU MDR requires manufacturers to take the generally accepted SOTA into account when designing and manufacturing medical devices. SOTA demonstrates what is currently generally accepted as good practice in technology and medicine. ISO 14971 also introduces SOTA as a new definition in its third edition (ISO 14971:2019). We can perform a thorough SOTA analysis for our customers by comparing their new product design and development efforts to competitor devices, standards or published data, generally acknowledged as state of the art. This analysis will help determine the extent to which the new device conforms to safety principles used for current SOTA devices and how acceptable the new device’s risks could be.
Post-market analysis
Regulators expect manufacturers to proactively collect and review post-market information, including HFE-related data. We help our customers conduct periodic post-market known-problems analyses and literature reviews to meet regulators’ post-market requirements. Notably, we can support additional HFE post-market efforts, such as designing and executing surveys and actual use usability tests and helping improve a manufacturer’s complaint system to facilitate the collection of use-related data.
Case study
“Engaging Emergo UL early has paid dividends since they raised items that were immediately actionable while others have continued to find their way into our future product roadmaps, and all of it laid important groundwork for development of our UE File.”
Fractyl
Human Factors Software
Optimal Product Usability Suite (OPUS™)
Emergo by UL’s cloud-based human factors engineering (HFE) platform leverages training, tools, templates and regulatory guidance to help you stay ahead in your HFE activities.
Request information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.