May 28, 2025
By Stuart R. Goldman
OCITE Helping Improve Your 510(k) Search Experience
The Office of Communication, Information Disclosure, Training and Education (OCITE) at the FDA's Center for Devices and Radiological Health (CDRH) has recently added six new sub-categories to the 510(k) Premarket Notification database, making it easier for users of this database to narrow their search when looking for information on medical devices that have been cleared by the FDA through a 510(k) pathway under a certain product code.
OCITE wanted to improve the user’s experience concerning the efficiency and accuracy of information retrieval related to cleared medical devices. By adding these sub-categories, OCITE aimed to provide more targeted and relevant search results, facilitating better access to regulatory and compliance information for industry stakeholders and the public.
OCITE adds sub-categories
The six new sub-categories for search are below and more than one sub-category can be searched simultaneously.
- Combination Products
- Cleared/Approved In Vitro Products
- Redacted FOIA 510(k)s
- Third Party Reviewed
- Clinical Trials
- Predetermined Change Control Plan Authorized
Information on the first five sub-categories of topics was always available on 510(k) cleared devices in the database if you located it during random searches of 510(k) Summaries, but there was no way to specifically search for this information until now.
Information about Predetermined Change Control Plan (PCCP)
The sixth sub-category of topics, “Predetermined Change Control Plan (PCCP) Authorized”, is for devices submitted under a 510(k) that also include a PCCP as discussed in the related FDA draft guidance document, Predetermined Change Control Plans for Medical Devices.
PCCPs also apply to other premarket submissions. As of May 22, 2025, only 42 PCCPs covering different product codes and review panels have been authorized under the 510(k) pathway.
Data about Freedom Of Information Act (FOIA) and clinicals
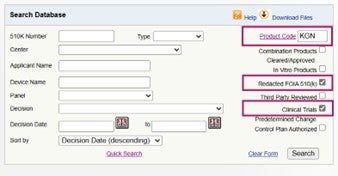
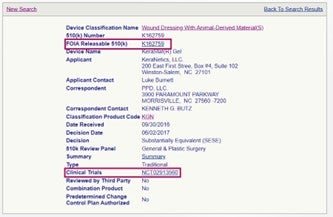
Two of the more popular sub-categories of topics that we often get asked about when working with device companies are “Redacted FOIA 510(k)” and “Clinical Trials” information on a competitor device, which is now made easy with the new sub-categories feature in the 510(k) database as seen in the example below using product code KGN (Wound Dressing with Animal-Derived Material(S)). This search yielded just one result as seen below. The hyperlinks to the Redacted FOIA 510(k) in the FDA’s 510(k) database and to the clinical trials information on the ClinicalTrials.gov website can be easily accessed with a single mouse click.

Concluding remarks
By adding the six new sub-categories to the FDA’s 510(k) database, OCITE has made the 510(k) search experience more efficient, accurate and easier for users of this database to look for information on similar medical devices.
The primary reason for this update by OCITE was to improve the user’s experience concerning the efficiency and accuracy of information retrieval related to medical devices submitted under a 510(k) pathway. By adding these sub-categories, OCITE aimed to provide more target and relevant search results to improve the search experience.
Our next article discusses searches in the European EUDAMED Device module.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.