February 20, 2024
We at Emergo by UL have seen an increase in labeling issues since the Date of Application (DoA) for the Regulations, Medical Device Regulation (2017/745, MDR) and In Vitro Diagnostic Device Regulation (2017/746, IVDR). The issues are identified during customs review which delays shipments of medical devices to intended recipients. These issues are communicated to the appropriate competent authorities who then inform Emergo Europe, as the European Authorized Representative and the manufacturer.
Additionally, Emergo by UL has been made aware of several issues identified by competent authorities: correct CE marking, appropriate languages, declarations and certificates and CE marking on the device.
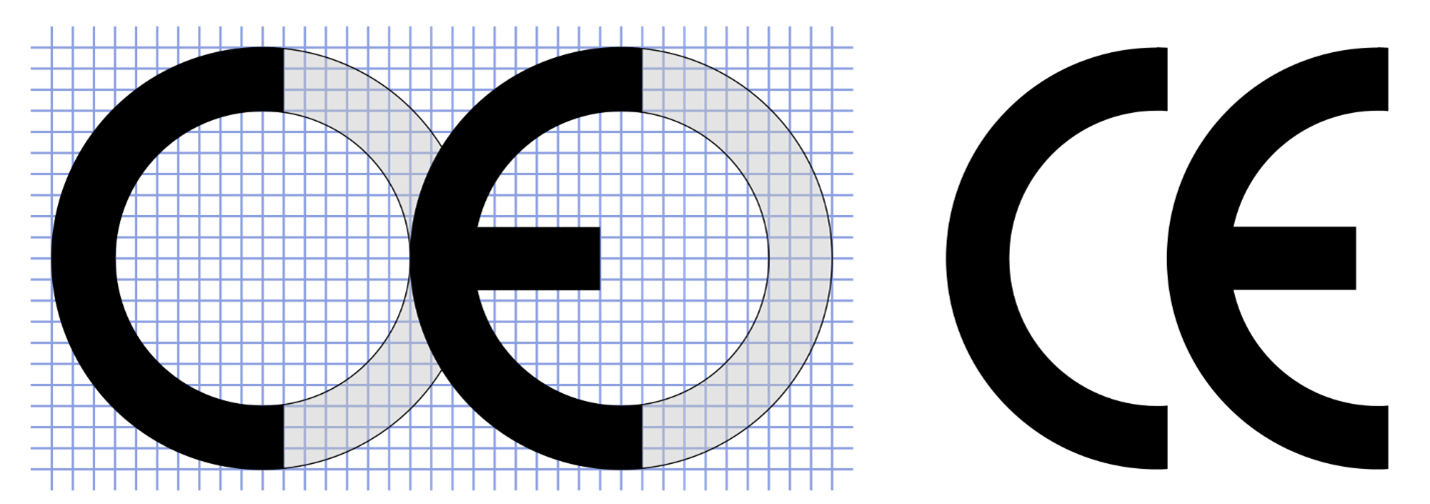
Correct CE marking
Many manufacturers are unaware that the China Export and CE marking logos are nearly identical and mistakenly use the China Export mark. Annex V of the Regulations illustrates the proper CE marking as noted below, left. The China Export mark, below, right, does not maintain the proper spacing between the C and E.

Language requirements
As mentioned in a previous regulatory update, the label and instructions for use (IFU) require translation into the official member state language where the device will be marketed, with few exceptions noted on the language requirement overview. The MDR and IVDR overview of language requirements for medical device manufacturers can be found on the European Commission website. Emergo by UL learned of a recent shipment that was denied access to the EU market, due to the untranslated IFU. The manufacturer was allowed to pay for its return. Otherwise, the shipment would have been disposed of.
Declaration of Conformity/NB issued certificates
The product in question must be listed on a Declaration of Conformity (DoC), which meets the requirements and should be on manufacturer letterhead. Note that if, under the scope of the MDD, IVDD or AIMDD, the DoC must be signed before the DoA. Any non-significant changes to the DoC can be listed on a supplement to the DoC. Certain competent authorities (CAs) who have rejected DoCs that have been revised post-DoA do not have a revision block indicating what was changed. Additionally, customs have questioned a certificate recently for products that did not appear to be listed under the scope of devices. Device family and trade names must be consistent throughout the documentation. This will help customs agents determine that the shipped device has proper CE marking.
Some markets require a copy of the DoC and NB-issued certificate, if applicable, to accompany the shipment.
CE marking on the device
Per the requirements of the Regulations, CE marking is required on the device itself or its sterile packaging, unless such affixing is not possible or warranted on account of the nature of the device (MDR Art. 20/IVDR Art. 18). In that case, the CE marking shall be affixed to the packaging. The IFU and any sales packaging must also bear the CE marking.
Concluding remarks
We at Emergo by UL have identified several common issues we’ve observed. Manufacturers must be compliant with the MDR and IVDR to obtain continued access to the European market.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.