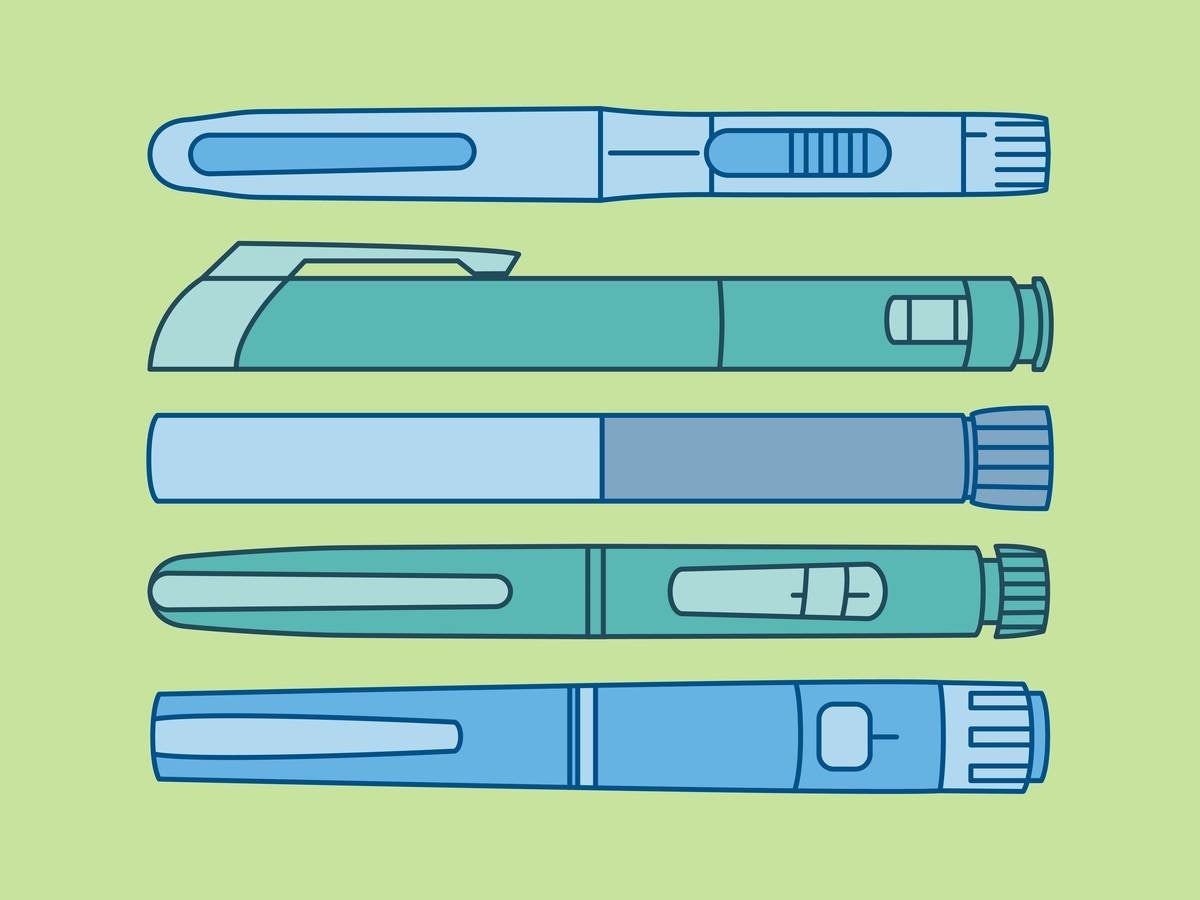
August 22, 2023
By Charlotte Wickham
Negative transfer is a common root cause for use errors and difficulties during device interactions. Negative transfer occurs when a user makes an assumption about how a device is going to work based on their experience with similar devices or similar device components.
It can be difficult to avoid errors caused by negative transfer because it can be difficult to know, or control, what products an intended user interacts with and what experience they are going to transfer to a new device. In this article, we will discuss how you can reduce the likelihood of errors due to negative transfer, and how you can use users’ prior experience to your advantage.
Negative transfer: a common root cause for use errors
Broadly speaking, negative transfer is assigned as the root cause for use errors when it is clear that the user(s) made an inappropriate prediction about how a device will work based on the performance of other devices that they are familiar with. Here are two real-life examples of negative transfer at work:
Device: Injection pen
- Use difficulty: The user attempts to remove a used needle by pulling it from the pen when the needle needs to be twisted.
- Participant-reported root cause: “With my usual pen, the needle just pulls off – it didn’t occur to me to twist this one…”
- Root cause: Negative transfer; the user has experience with pen needles that pull off, and therefore assumed this needle would pull off.
Device: Electronic autoinjector
- Use difficulty: The user attempts to remove the cartridge by pulling at the cartridge cover when the cartridge cover needs to be unlocked first.
- Participant reported root cause: “I thought I could just open it. That bit of plastic looks like the battery cover on my TV remote, and I can open that.”
- Root cause: Negative transfer; the user thought that the cartridge cover looked like a battery cover and thought that it would open in a similar way.
As illustrated, users’ experience can be transferred between similar medical devices (example one), which can cause problems when a user moves from one medical device to another similar one. It can also occur when users make assumptions about how aspects of a medical device will perform based on presumed similarity to everyday objects (example two).
Preventing negative transfer in the device design process
Below, we share some thoughts on preventing negative transfer.
Determine the similarity to other devices. During device development, conduct research to establish which devices your intended users will already be familiar with, and consider how that familiarity might affect their interactions with your device. Overall, understanding and meeting your users’ expectations for device interaction can help reduce the likelihood of use and difficulties and optimize user satisfaction. During device development, conduct research to establish which devices your intended users will already be familiar with, and consider how that familiarity might affect their interactions with your device. The extent to which users anticipate differences can depend on different characteristics, for example:
- Simplicity/Complexity of interactions. With simple interactions (e.g., removing/attaching components) users might expect your device to work similarly to other devices. On the other hand, with more complicated interactions, users might anticipate some differences between similar devices and will be more likely to explore the device carefully and check the labeling.
- Simplicity/Complexity of the device. Users might expect something simple, like an inhaler, to operate in a similar manner to other inhalers they have encountered. For something more complex, like an insulin pump, they might be more likely to explore the layout and features and rely less on prior experience.
- Similarity between existing devices. If a user has experience with various brands or versions of the same type of device (e.g., an autoinjector) and has found they generally operate in a similar manner, they are more likely to be “thrown” by a new version that breaks the mold.
Optimize the readability of your labeling. Negative transfer often occurs when users are operating a device via “guesswork,” i.e., without the assistance of labeling (such as instructions for use [IFU] or quick reference guides [QRG]). By ensuring your labeling is easy to read and accessible, you can limit negative transfer by preventing the trial-and-error approach. Pay particular attention to steps where you know your device will operate differently from similar devices, and ensure this step is clearly outlined and/or highlighted.
Provide consistency within your own products. At a minimum, control what you can by ensuring consistency, where possible, of the user interface design across your products. For example, look for common approaches to component layout, task flow, iconography, and part attachment and detachment. Users are more likely to expect consistency when moving between products belonging to the same brand, and you can help ease the transition by providing a user experience with which they will be familiar. If you are updating an existing product – such as by providing a software update – you have the benefit of knowing how your users will expect the new version to behave and can use this knowledge to prevent use errors and difficulties.
Using users’ prior experience to design user-friendly medical devices
Users’ prior experience can also have a positive effect on user interaction, and this is something you can leverage.
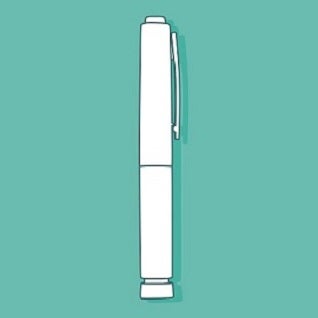
Consider the two medical devices that are illustrated below: an insulin pen-injector and an epinephrine autoinjector. In the absence of labeling, which way up should these devices go? What parts of these devices are removable? Do you need to press something to get the medication to come out?

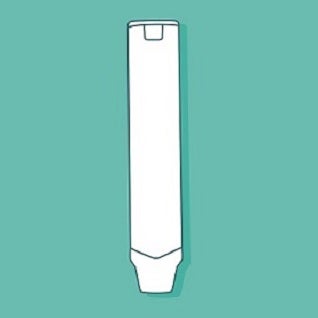
These questions are easier to answer for the pen-injector than the autoinjector, and the reason for this is simple. Pen-injectors look like pens, and we know how a pen works. We know that the part with the pen-clip is the lid, and we know that the lid needs to be removed before the pen can be used. The actuation button on the end is familiar as well, because it is similar to the button some ball-point pens have to get the nib to pop up. Moreover, by leveraging the appearance and functionality of a simple, everyday product and applying it to a medical device, you can provide quick answers to the questions that pop-up during the first use, such as, “Which way up does this thing go? Is that a lid? Where is the button?”
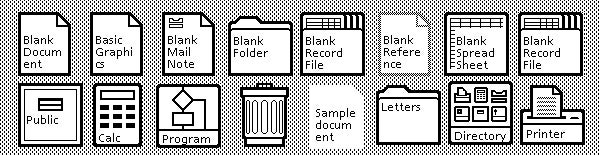
Computer operating systems and programs make good use of this by using everyday objects within their user interfaces with terms such as desktop, file, folder, copy, and paste. The first consumer computer with a graphical user interface (GUI) went with this approach in 1981, using icons that looked like envelopes, recycling bins, calculators, and paper folders (shown below). Because it works so well, we still see it now.
Conclusion: optimizing medical device usability
To help your team avoid negative transfer, ensure that you drive successful device development with your user research by looking for the potential ways negative transfer could reveal itself in your future designs. Conversely, consider ways of leveraging your users’ experience with other devices – both medical devices and everyday objects. Such “positive transfer” – might enable you to better communicate proper use in a clear, quick manner.
Contact our team to discuss more ways to optimize the usability of your medical device.
Charlotte Wickham is Senior Human Factors Specialist at Emergo by UL's Human Factors Research & Design division.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.