Save time and minimize errors in your regulatory documentation
Smart Builder walks you through regulatory documentation for Brazil’s Registro Route, Mexico Equivalency and Standard Route, and Europe’s MDD, MDR, and IVDR.
Comply with the EU MDD, MDR, IVDR, and MEDDEV 2.7/1
Smart Builder provides a clear structure to create medical device technical documentation compliant with MDD and MDR, with the potential to seamlessly transition between the two regulatory schemes. Get a head start on preparing for IVDR compliance with dedicated Smart Builder for your Technical Documentation File, Performance Evaluation Plan, and Performance Evaluation Report.
Create a living document for your technical documentation and insert references to your QMS system for appendices documents for seamless updates. Copy and import features that will sync between projects to make it easier to convert from MDD to MDR.
Get it right the first time with intuitive form completion tools

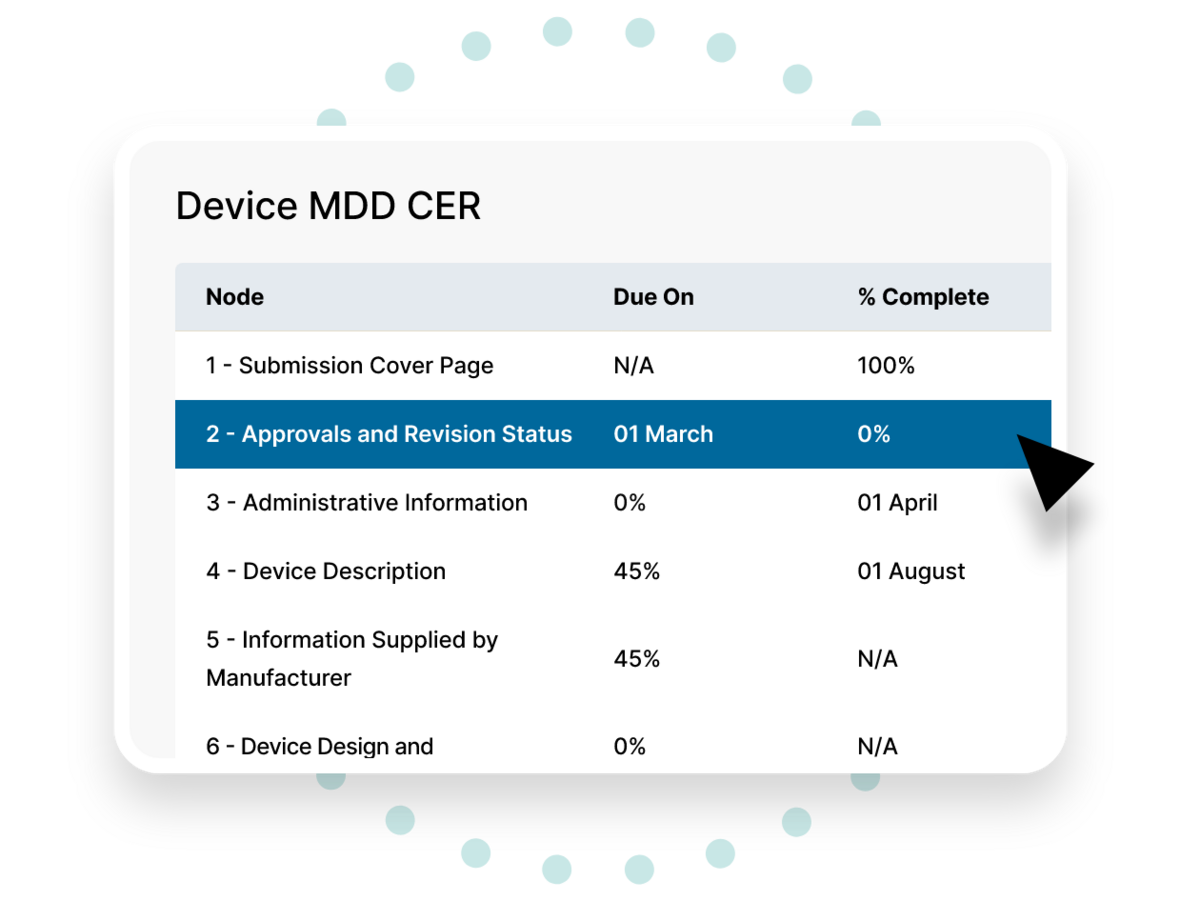
This headache-free system for building and maintaining your registration dossier helps you avoid errors and inconsistencies that risk delaying or jeopardizing your market access.

Advance through every section with guidance on what each field should contain. The progress bar provides an at-a-glance snapshot of how far along you are.

Key textual components like the product name and indications for use can be recorded as keywords and applied throughout the document to ensure that they remain consistent.
Share notes and insights with the comment system
RAMS Smart Builder makes it easy to coordinate edits and share progress with teammates. Track your progress, catch typos, and note outstanding needs.

Commenting gives you the ability to customize your project workflows to the dynamics of your team. Get the necessary information in one place where all the right people can see it.

Smart Builder also allows you to tag individual coworkers in your comments and notify them via email of any changes or assignments.
Generate a document that you can send right away

RAMS Smart Builder arranges your forms into a professional document pursuant to applicable regulatory requirements — there’s no need to spend further time on formatting.

Export a PDF of your completed submission that is ready to send without any hassle.
Get worldwide market support

Access growing Latin American markets
- Material and equipment included for Brazil’s Registro Route
- Supports English language input to accommodate preparing your documentation in Portuguese
- Generates a Letter of Authorization template
- Mexico Equivalency and Standard Route available for U.S. FDA, Health Canada, and Japan MHLW

Global representation through one point of contact
Medical device regulations in most countries require foreign manufacturers to appoint an in-country representative. With offices around the world, Emergo by UL can serve as your in-country representative in the U.S., Europe and beyond.
Learn about in-country representation
Simplify your regulatory documentation
Request your personalized RAMS Smart Builder quote today.
Simplify your regulatory documentation
Request your personalized RAMS Smart Builder quote today.