May 8, 2024
By Daniela Sanchez Gálvez and Dicsy Solano
This is our fourth update on the recently released draft standard for medical device labeling published by Mexico’s medical device regulator COFEPRIS, NOM-137-SSA1-2024.
Background on NOM-137-SSA1-2024
COFEPRIS released (March 12) a new draft of the NOM for Medical Device labeling, NOM-137-SSA1-2024. We posted part 2 and part 3, to further clarify the proposed medical device labeling standard. In this last part, we discuss the new symbols and share the descriptions.
Identification of the medical device manufacturer
The most remarkable changes in this draft are:
- The explanation that the country of origin of the medical device is not to be confused with the real manufacturer, which may differ. The country of origin was required in the previous NOM and while it has not been officially defined to date, it is understood to be where the devices are shipped from before import into Mexico.
- Abbreviations are now permitted within the address.
- Medical devices imported as samples must be identified according to the manufacturer’s QMS and shall include the statement “Prohibited for sale”.
Performance of the medical device
The instructions for use (IFU) must state which precautions or other measures the patient should take if the performance of the medical device fails, as well as those needed at the end or near the end of the shelf life of the device.
Specific labeling requirements for medical devices
In addition to the general requirements already addressed in our past regulatory updates, the following need to be considered:
- Medical devices intended exclusively for public health and social security institutions, will need to state, "Not for sale" or "Property of the health authorities” in at least one of the labeling elements.
- There are updates concerning medical device kit labeling, such as the need to include each of the registration numbers of the components, together with the information necessary to comply with the medical device label requirements. It is worth noting that the expiration date for the kit must be shorter than that of the components within.
- Medical devices that contain cells, tissues or derivatives of human or animal origin, or any drug or medication, must indicate this on the label including the quantity, concentration thereof and whether it will be in direct contact with the patient.
- IVDs should include information on signs to dispose of the device, such as degradation or the maximum number of reuses.
- Whenever a medical device that, due to its nature, size or design, cannot contain all of the information required by the NOM, a secondary label or the IFU must include the remainder.
Labeling requirements for SaMD
Although software as a medical device (SaMD) has been officially regulated in Mexico since July 10, 2023, details about labeling requirements were not defined until the publication of this new draft of the NOM, which is as follows:
- Generic name
- Commercial name
- Registration number
- SaMD identifier (catalog, reference, model or version number)
- Warnings, precautions or measures (symbology applies)
- Indication for use
- Instructions for use (IFUs) or user manual
- Manufacturer
Whenever there is no physical form or packaging for the SaMD, the label should be electronically available.
Again, when we refer to SaMD, the acronym proposed in the NOM is ScDM.
Specific labeling requirements for medical devices for the general population
The IFU destined primarily for the general population or layperson use must be presented appropriately. The intended use can be stated in a simplified way, with some details being omitted if they do not alter the risk or performance of the device, as confirmed by risk management.
IVD self-tests must be identified as such and defined as to whether they require the participation of a third-party care provider.
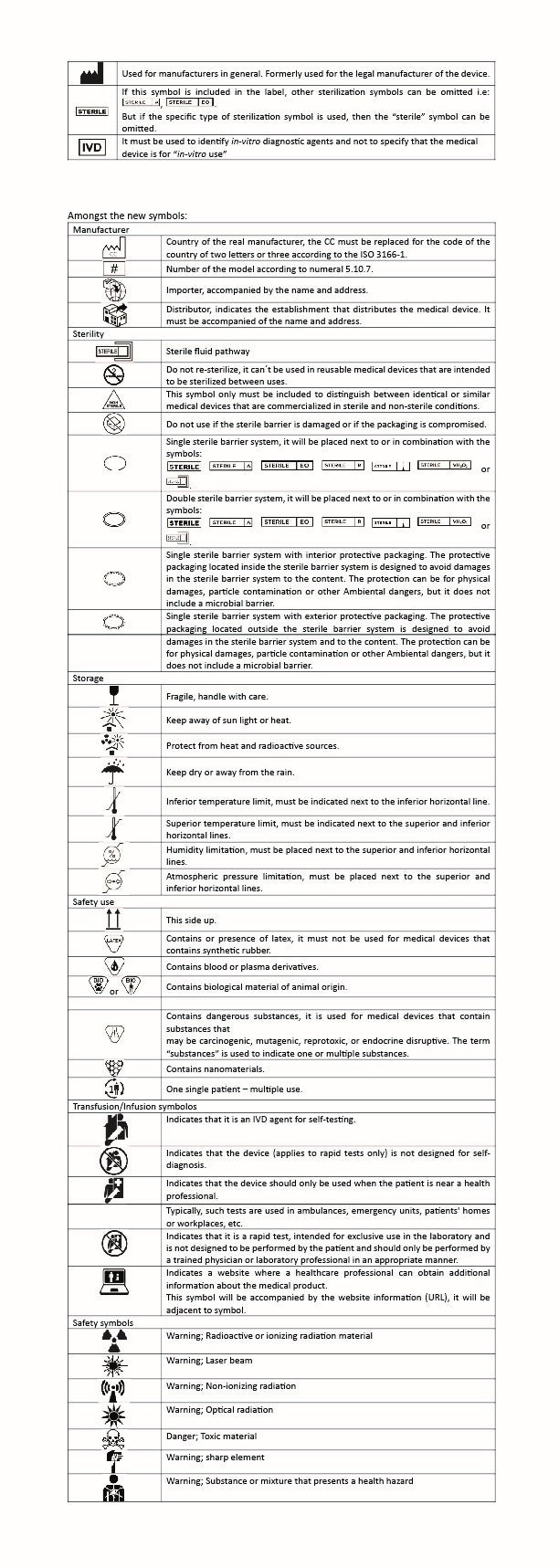
Appendix A (symbols)
The new Appendix includes an extended list of approved symbols.
Some changes in symbols compared to the current version of the NOM.

Concluding remarks
The new draft for the NOM for Medical Device labeling, NOM-137-SSA1-2024, contains several new definitions aligned with the most updated Mexican and international standards and regulations. It’s currently open for consultation.
We at Emergo by UL will share updates once the official version of the standard is published. Significant changes are not expected; however, this revision represents a comprehensive change and significant additional requirements for medical device labeling.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.