August 19, 2025
By Razel Cada, Beth Pugh and Evangeline Loh
The regulator in Switzerland, Swissmedic, continues to advance development of the Swiss Database on Medical Devices, Swissdamed: ACT module (registration of economic operators), a publicly accessible platform, and UDI module (registration of devices). Most recently (August 18), Swissmedic shared a revised timeline for Swissdamed compliance and announced the availability of the UDI Devices module.
While the Swiss medical device regulatory system (Medical Devices Ordinance (MedDO; SR 812.213) and the Ordinance on In Vitro Diagnostic Medical Devices (IvDO; SR 812.219)) leverages compliance with aspects of EU legislation, unfortunately, there is no interface between Swissdamed and the European database EUDAMED.
Swissdamed updates
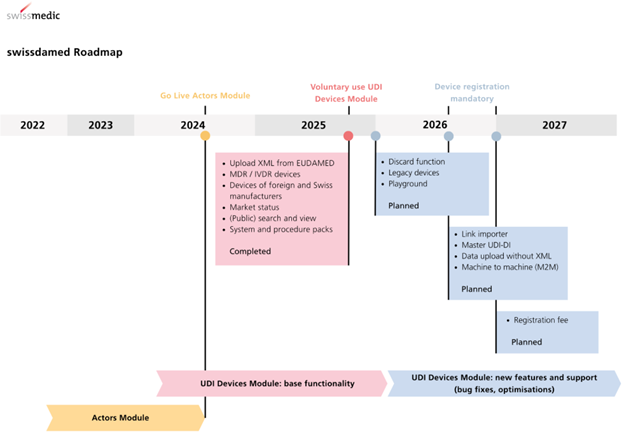
The Actors module has been in use since August 2024. The UDI Devices module has been available for voluntary use since August 2025. Mandatory use of which will take effect on July 1, 2026, for devices, including systems and procedure packs. With a six-month transition period, registration in the UDI Device module will be mandatory on January 1, 2027.
If a serious incident, Field Safety Corrective Action or trend is relevant for a device or system/procedure pack after July 1, 2026, registration would be immediate, without a transitional period.
Swissdamed guidance
As part of this (August 15), Swissmedic released a Swissdamed User Guide UDI Devices Module (BW630_40_841), and revised the Swissdamed User Guide Actors (BW630_40_001).
Two quick guides were also made available: to facilitate public searches for actors and devices and for registration in the UDI Devices module.
Timeline extended for UDI Module compliance (registration)
Development of medical device databases requires more time than expected, as evidenced by Swissdamed and EUDAMED, as well as SIUD (Brazil UDI database). In Switzerland, the UDI Module becomes mandatory at the end of 2026, unless the device becomes subject to post-market surveillance activity, in which registration of the device or system/procedure pack becomes immediately required after July 1, 2026.
Our Emergo by UL experts will continue to monitor and publish updates on these developments.
Image credit: Swissmedic

Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.