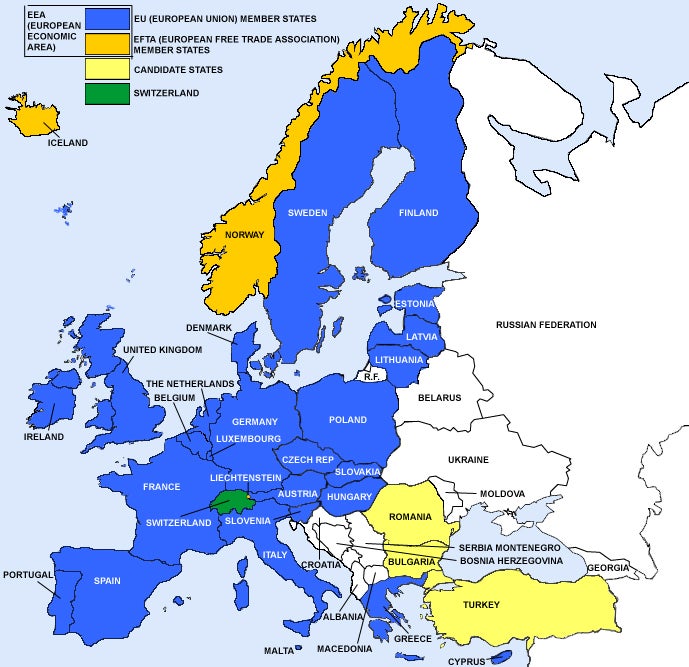
The European Union comprises 28 countries that require CE Marking. Three additional countries (Norway, Iceland, Liechtenstein), although not officially part of the European Union, are signatories to the European Economic Area (EEA). Switzerland is not an EU member nor a signatory to the EEA, but they have transposed the Medical Devices Directives into their national law and these countries require CE Marking.
We can assist you in obtaining CE Marking so you can sell your medical device in any of these European countries.
Request more information from our specialist
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.







