Utilize medical device product testing early to accelerate development
Early-stage design evaluations are a critical opportunity for medical device developers to optimize user interfaces and reduce the risk of use errors before formal usability testing. At Emergo by UL, we help manufacturers fine-tune their designs through expert reviews, user-informed walkthroughs and concept-level feedback to help early iterations align with human factors engineering (HFE) for medical devices best practices and regulatory expectations.
With decades of experience and leadership in shaping device usability guidance, Emergo by UL’s Human Factors Research & Design (HFR&D) team supports customers in identifying issues early when improvements are most feasible and cost-effective. Whether seeking feedback on an early prototype or revisiting a product line with known user challenges, our early design evaluation services give you the actionable insights you need to move forward confidently.
The role of HFE in medical device development
Before a medical device reaches production, numerous design decisions shape how users interact with it. The earlier those decisions are assessed via medical device testing through a human factors lens, the more effectively your team can prevent design-related use errors and reduce costly revisions later in development.
Early design evaluations can:
- Uncover user interface elements that may lead to confusion or misuse.
- Provide objective, expert analysis based on established usability principles.
- Align product design with international standards and regulatory guidance.
- Validate early concepts through structured input from representative users.
- Lay the groundwork for a successful formative testing plan or redesign strategy.
Rather than waiting until formative usability testing to uncover critical issues, an early evaluation can help your team focus time and resources on the most impactful refinements, helping to accelerate your path to regulatory approval and market success.
Our approach to early design evaluations and medical device product testing
We tailor each evaluation to your project's stage and specific needs. Drawing from our work on hundreds of device types, like combination products and surgical systems, we combine deep regulatory knowledge with practical, real-world usability insights. At this stage, our medical device testing may include:
1. Design critiques or heuristic reviews
Design critiques, also known as heuristic evaluations, are expert-led reviews of your product’s user interface, including physical, digital or labeling-related aspects, against established usability principles. These reviews are fast, focused and ideal for identifying early-stage usability issues.
Our team uses metrics derived from peer-reviewed literature, international standards and extensive hands-on experience to assess design elements such as:
- Control placement labeling.
- Menu structure and navigation logic.
- Instructions for use (IFU) clarity.
- Physical affordances and feedback mechanisms.
- Interface consistency and visual hierarchy.
Our evaluations not only flag potential issues but also highlight areas where your design already performs well. The resulting feedback can inform design sprints, guide iterations and support documentation for internal design history files (DHFs).
2. Cognitive walkthroughs with representative users
To go beyond expert opinions, we also offer cognitive walkthroughs, which are structured sessions where representative users interact with early-stage concepts, such as low-fidelity mockups, interface flows or paper prototypes, and verbalize how they would use the product to complete key tasks.
Cognitive walkthroughs can reveal:
How users interpret cues and instructions.
Points of confusion, hesitation or incorrect assumptions.
Differences between designer intent and user expectations.
Opportunities to improve overall task flow and usability.
Unlike full usability tests, which often require high-fidelity prototypes and formal protocols, cognitive walkthroughs are lightweight and flexible, making them ideal for early development phases or exploring multiple design directions.
Real-world impact: case study
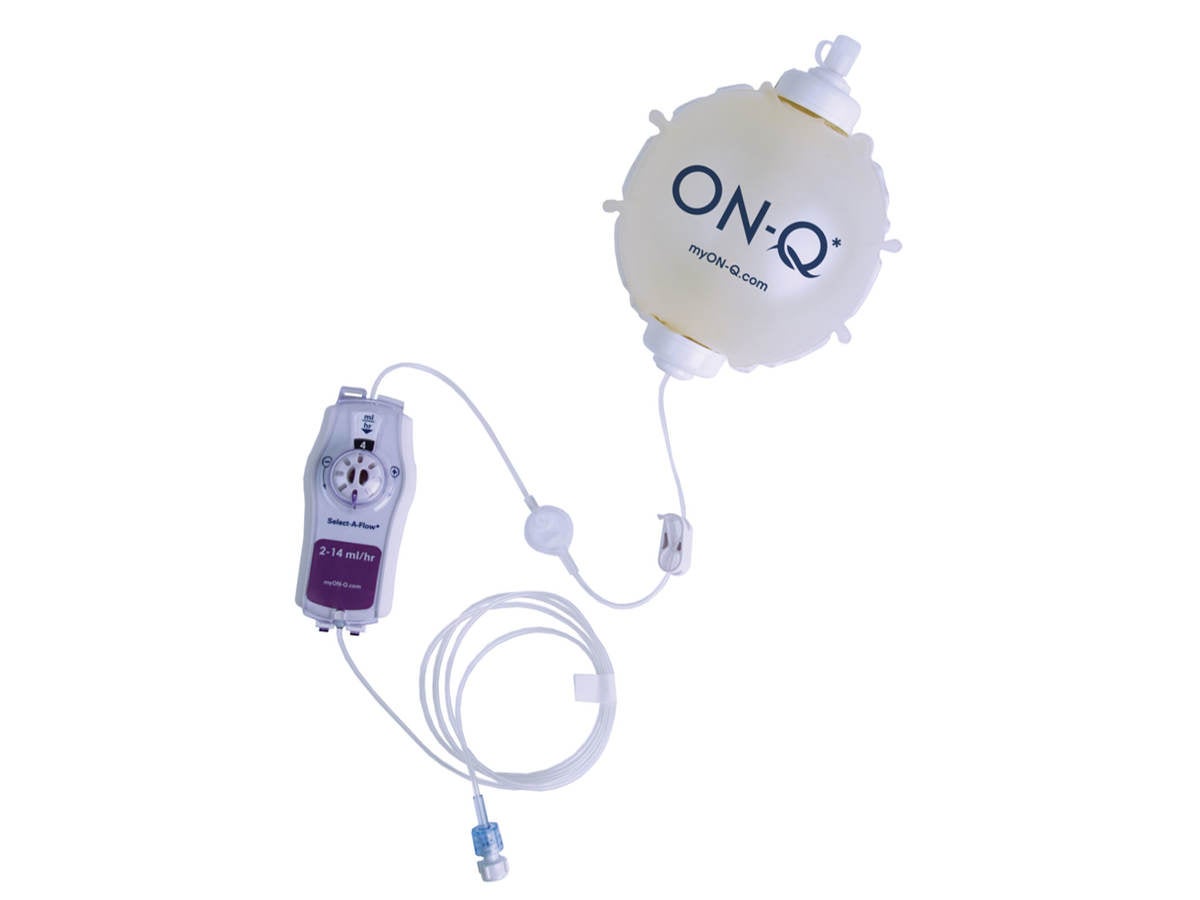
Refining flow control in an infusion pump: Avanos ON-Q* with SELECT-A-FLOW*
When Avanos Medical, Inc. sought to enhance the usability of its ON-Q* pump with SELECT-A-FLOW* , the company chose the Emergo by UL human factors team to conduct early evaluations. The pump’s new feature, a flow rate selector, introduced a novel interface element that needed to be intuitive for clinicians and patients.
Our team began with a heuristic review, identifying potential areas of risk related to flow rate labeling and user control feedback. We then facilitated user-informed walkthroughs with representative clinicians to understand how they interpreted and interacted with the new design. Their insights revealed discrepancies between intended use and real-world behavior, prompting valuable design refinements.
These early-stage evaluations helped Avanos refine key interface elements before formative testing began, saving time, reducing development costs and supporting safer use by end users. Read “Innovating Pain Management: Avanos' ON-Q Pump” for more information.
When to use early design evaluations
Whether you’re in the discovery phase or mid-cycle on a next-gen device, integrating human factors thinking early helps produce safer and more effective products from the start. Early evaluations are especially useful during:
Concept development
Evaluating mockups and wireframes before prototypes are built.
Pre-formative testing
Identifying quick wins that reduce test failures and user frustration.
Regulatory preparation
Gathering usability rationale for regulatory submissions worldwide.
Advantages of working with Emergo by UL
Choosing Emergo by UL for your early design evaluations brings several benefits:
Deep experience across device types
Our team has worked on a wide variety of products, including:
- Drug-device combination products.
- Home-use and wearable medical devices.
- Robotic and traditional surgical systems.
- Software as a medical device (SaMD).
- Connected care platforms.
We understand the distinct needs of each category and apply that insight to every evaluation.
Recognized human factors leadership
Emergo by UL has played a key role in advancing the field of medical device usability, authoring widely used references like books, white papers, insight articles, webinars and training. Our insights often shape internal design processes and external regulatory frameworks alike.
Flexible and collaborative support
Depending on your workflows, we can work as an extension of your internal design team or provide an outside perspective. We offer:
- One-time reviews and walkthroughs.
- Ongoing consultation during iterative design.
- Cross-functional sessions with different teams.
Ready to get started?
Early design evaluations can make the difference between a smooth validation process and costly last-minute redesigns. Emergo by UL’s human factors experts help you move from idea to implementation with user-informed medical device testing feedback. Let’s talk about your goals and how we can support your team. Contact us to discuss your project.
Related content
- A Focus on Usability Testing Protocol Development: Components of Evaluation Activities
- Risk Analysis for Design and Innovation
- Design and Evaluation of Electronic Instructions for Use (eIFUs)
- Knowledge Task Testing to Validate Instructions and Warnings
- TalkingPoints: Human Factors and Usability of Medical Device News