The discovery phase in medical device development
Begin your medical device development with discovery to uncover opportunities, identify user needs and set the right direction for success.
Applying a collaborative approach, we can help you chart a path forward. Perhaps you want to explore possibilities to enhance the user experience through innovative solutions. Perhaps you need a defined strategy to guide project prioritization or align business stakeholders to a common vision. Perhaps you want to survey the market landscape for the next big trends or determine where you can create a new trend. In all cases, understanding the opportunity space — including unmet user needs, underserved markets or emerging trends — will be key to your success.
We recommend that you diligently study the area of interest before you start to create, and we can serve a crucial role as your partner in this phase. Specifically, we can provide the right cross-functional team from our large pool of researchers, designers, human factors specialists, engineers and regulatory experts to clarify the following:
- Outstanding user needs and unfulfilled desires
- Development opportunities and value propositions
- Competitive landscape and analysis
- Trend reports
- Exploration of early solutions, including ideation and evaluation
- Use-related risk potential
- Regulatory pathways and strategy
Our comprehensive discovery process
Research user impacts, conduct collaborative design sessions, evaluate concepts, refine ideas and align with stakeholders for successful development.
Explore
Research and analyze impacts to the user experience, including safety, scope, feasibility and unresolved user needs.
Diverge
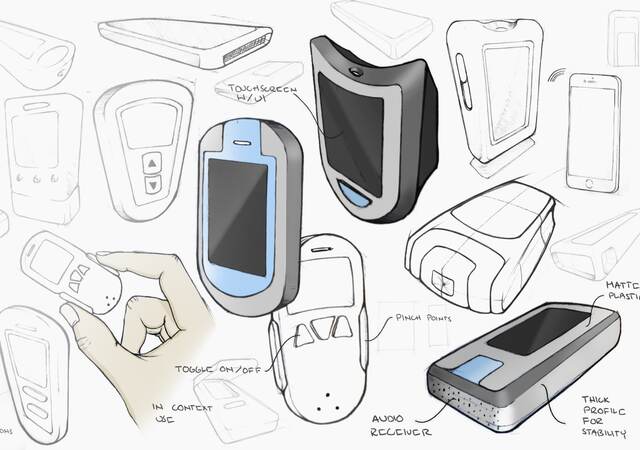
Lead collaborative design sessions to explore the “design space” and, ultimately, produce a wide range of practical to blue-sky concepts.
Evaluate
Present concepts, such as sketches or prototypes, to prospective users and stakeholders to collect impressions and recommendations.
Converge
Select and refine the most promising concept(s) as a starting point for focused product development or broader product strategy.
Confirm
Conduct a cross-functional review of concepts with key stakeholders to confirm feasibility and gain alignment on selected directions.
This review might determine the need for further research or development to inform decision-making, noting that a design iteration is not a negative outcome but an opportunity to drive excellence.
Diverse applications of our discovery activities
Our discovery activities span various applications, from patient monitoring systems to infant nebulizers, providing tailored support for unique challenges.

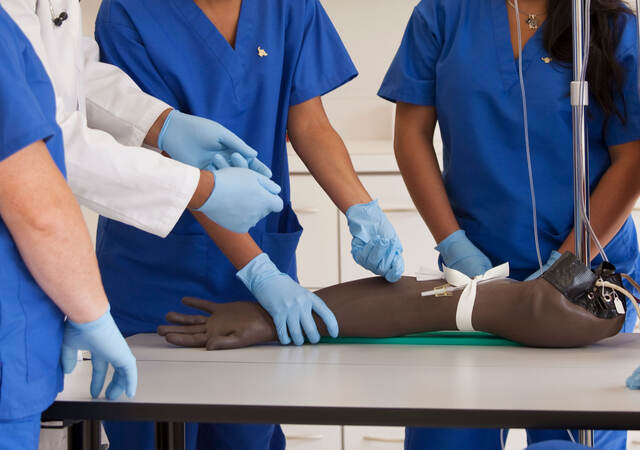
Spending days observing the use of telemetry technology on a medical/surgical unit in a hospital to help determine the safest course of future patient monitoring system development efforts.
Working with parents of infants to determine the desirable characteristics of an infant-sized nebulizer.
Conducting observations and interviews of EMTs and paramedics to determine the desirable features of a patient monitor/defibrillator.
Conducting observations and interviews of clinicians and patients in dialysis clinics to help shape a vision for home dialysis solutions.
Examining treatment rooms within several endoscopy units and interviewing physicians, nurses and anesthesiologists to determine challenges within and opportunities to improve specific treatments, with a focus on key features of a new piece of capital ablation equipment.
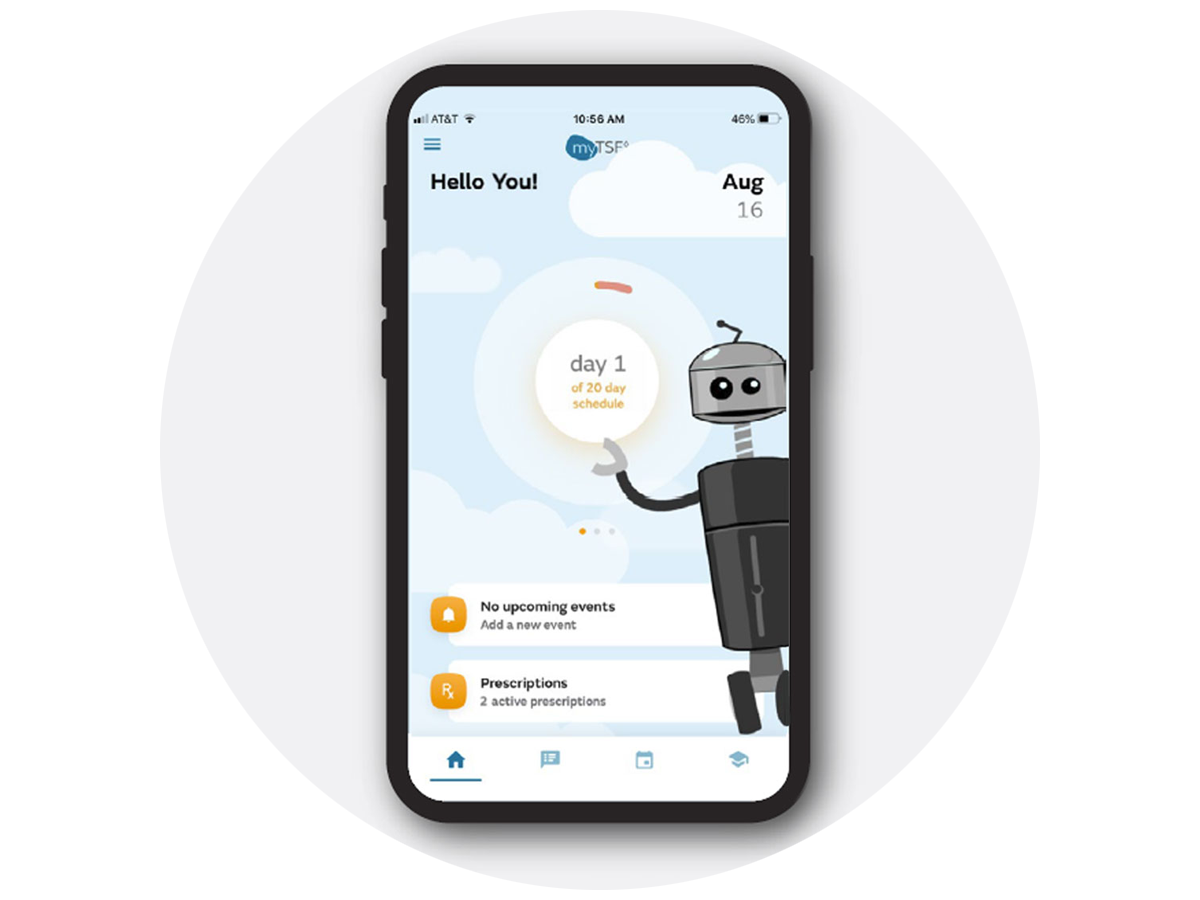
Case study
“Wearing a TSF for several months can be a daunting experience. The Smith+Nephew vision was to create a companion for the patient on their TSF journey. The team at Emergo by UL immersed themselves in the world of TSF and breathed life into that vision.”
Jean Ryan, senior global product manager, Trauma
Smith+Nephew