Expert guidance to navigate human factors challenges
Emergo by UL offers comprehensive human factors engineering (HFE) consulting services tailored to the medical device and pharmaceutical industries. With over two decades of experience, our team assists clients in developing strategies that can enhance device usability, safety and regulatory compliance.
Extensive industry experience
Our consultants have contributed to the success of hundreds of medical products. We provide support across various domains, including:
Drug-device combination products
These include injection devices, inhalers, dermal patches and on-body injectors.
In-vitro diagnostic (IVD) devices
This category includes laboratory-based equipment and at-home testing kits.
Software as a medical device (SaMD)
Desktop and mobile applications for clinical diagnosis and patient care.
Surgical devices
Including robotic-assisted and traditional tools used by various specialists.
Home-use medical devices
Combination products, enteral feeding and drug infusion pumps, cardiac monitoring equipment and dialysis systems.
Professional healthcare devices
Infusion pumps, patient monitoring systems and many others.
Robotic surgical systems
Systems that are intended to be operated by multiple users simultaneously.
The support provided for each device can cover the entire product lifecycle, including development, regulatory compliance and aftermarket monitoring. Our teams’ breadth of experience positions us to address diverse HFE challenges effectively.
Comprehensive regulatory strategy support
Success with global medical device regulations requires a nuanced understanding. Our consultants stay abreast of evolving regulatory landscapes to provide informed guidance. Emergo by UL offers strategic support to align your HFE activities with regulatory expectations, including:
Developing use specifications
To guide design in accordance with IEC 62366-1:2015 Medical devices – Part 1: Application of usability engineering to medical devices.
Conducting use-related risk analyses
We work with customers throughout the design process to identify potential user errors and mitigate risks.
HFE and usability report preparation
We can assist with any stage of the report production process and submission for regulatory bodies around the world.
Regulatory feedback response
We assist in addressing concerns raised in communications such as Additional Information and Complete Response letters.
Regulatory meeting facilitation
We can help with meeting initiation and provide expert insights during agency interactions.
Specialized human factors training
Our consultants also work with teams to build on existing HFE knowledge that can be immediately applied to assist with product development. Our specialized training is designed to help build internal competencies and support ongoing product development efforts where you need it the most. Emergo by UL’s customized training programs have covered:
Fundamentals of human factors engineering
This training can help fill knowledge gaps between mechanical and software teams.
Regulatory requirements
In-depth insights into the regulations your product needs to follow can help a variety of different teams.
Usability testing methodologies
We can look at methods currently being used and train on new ones that could help improve development.
Documentation practices
Instead of relying on a third party for your documentation, we can help you bring it in-house.
Flexible consulting engagements
Our goal is to support your objectives by effectively adapting our services to fit your project’s scope and timeline. Recognizing that each project has unique needs, Emergo by UL offers flexible consulting services, including:
Advisory roles
We provide guidance on specific HFE aspects, from product development to aftermarket monitoring.
Collaborative projects
We work alongside your team throughout the development and submission processes.
Independent assessment
Our team can conduct evaluations and provide actionable recommendations.
Case study
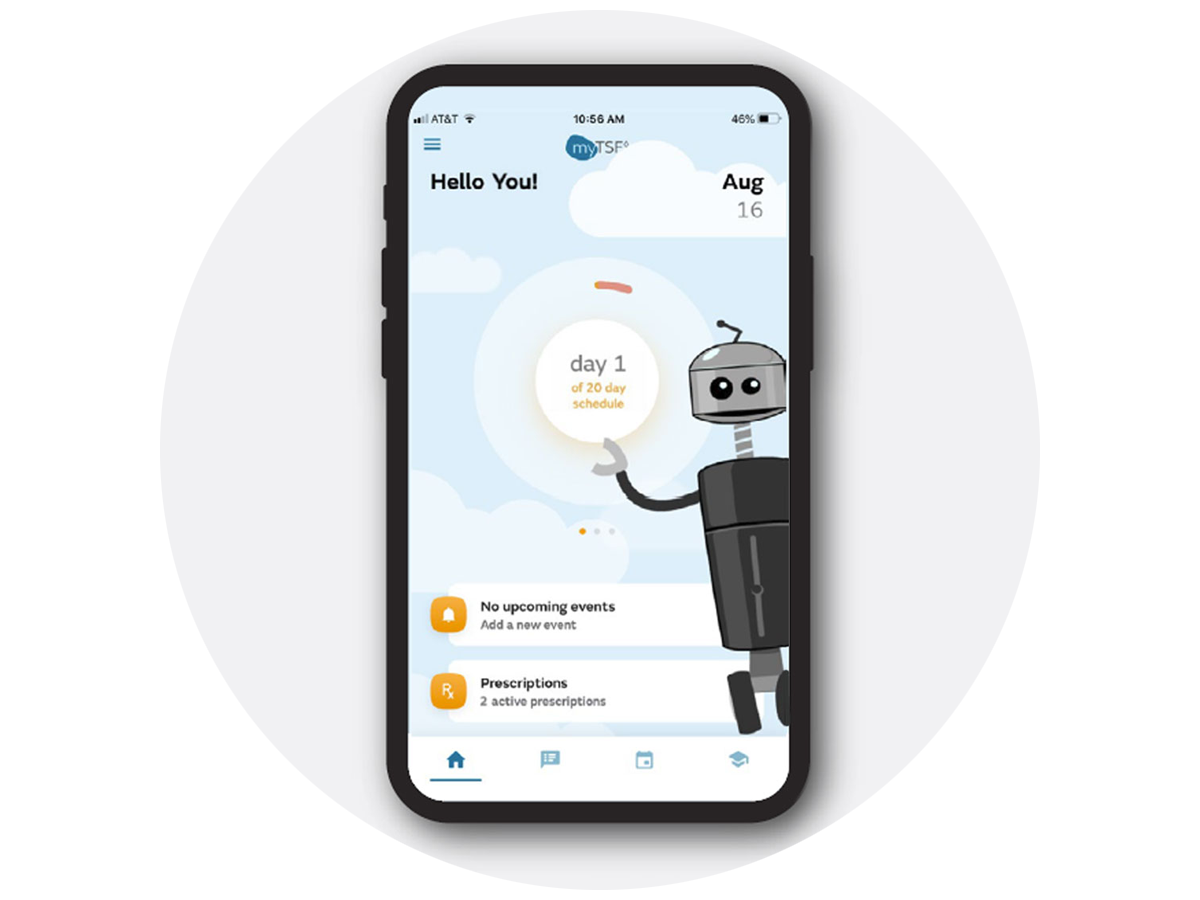
Breathing life into a new user experience
Emergo by UL worked with Smith+Nephew to enhance the usability of the myTSF™ mobile app, designed to support clinicians using the Total Surgical Flow platform. Our human factors experts conducted research to understand users’ contexts and expectations, then translated the findings into design insights and development guidance. Their work resulted in an intuitive app experience that supports efficient and accurate use in fast-paced surgical settings. This project reflects our commitment to shaping user-centered solutions that align with clinical workflows and user needs.
Connect with Emergo by UL
Learn more about our human factors engineering consulting services and how we can support your medical device development.