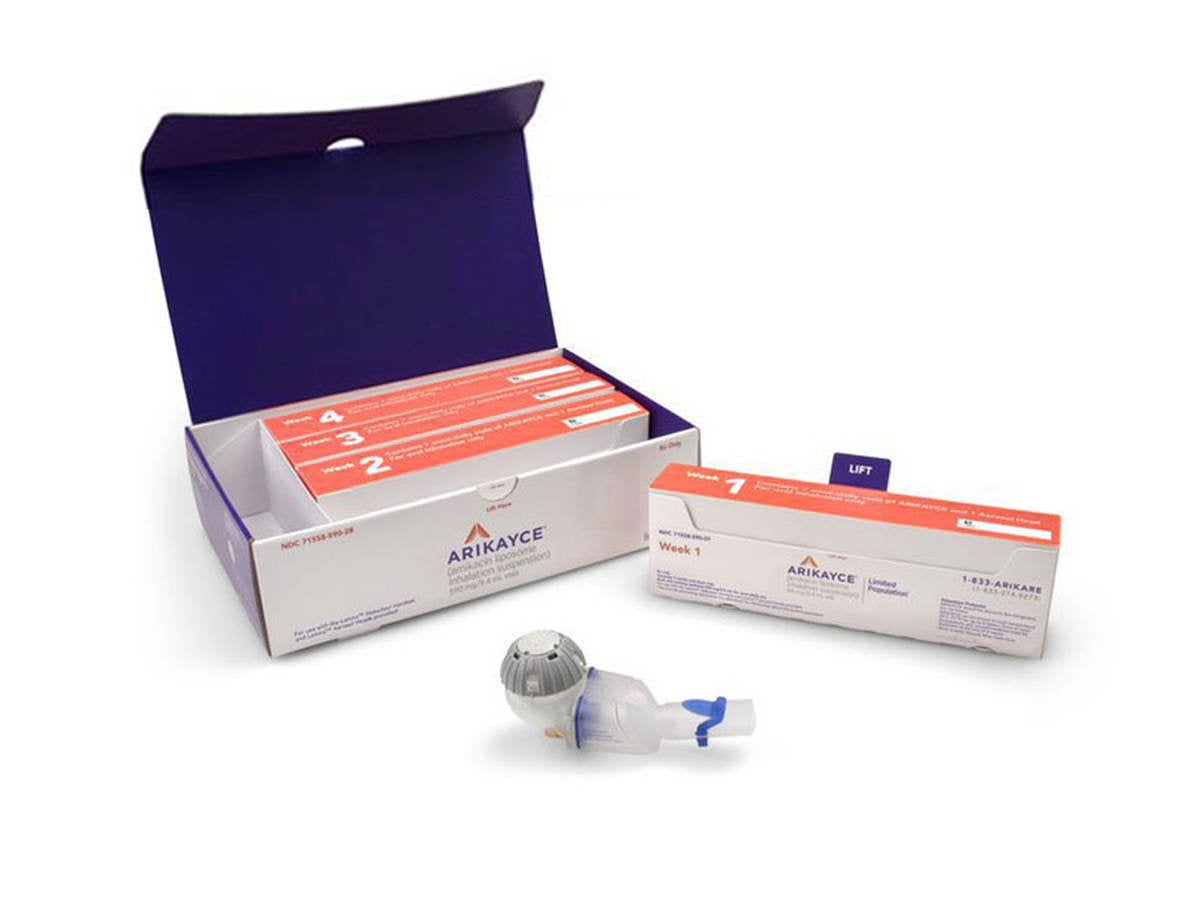
Device description
ARIKAYCE® (amikacin liposome inhalation suspension) is the first and only therapy approved for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen for adult patients who have limited or no alternative treatment options. ARIKAYCE is administered once daily using the Lamira® Nebulizer System, which is based on PARI Pharma's eFlow® platform.
Challenge
As Insmed prepared to submit a new drug application (NDA) to the U.S. Food and Drug Administration (FDA) for ARIKAYCE, the company decided to conduct HF validation testing of the Lamira® nebulizer system to ensure optimal usability of the device. Insmed engaged Emergo to ensure that the HF validation test was properly scoped, conducted smoothly and that various predecessor tasks were completed.
Solution
We worked closely with Insmed to develop the use-related risk analysis, conduct the formative and HF validation usability tests, advise on further mitigations, and support discussions with FDA regarding various aspects of the requested HF validation usability test (e.g., user groups, sample size, training). Our work culminated in a large-scale (N = 92) HF validation test including trained and untrained representatives of three distinct user groups that generated data to support Insmed's NDA submission.
Impact
In close collaboration with Insmed, we performed a wide range of HFE tasks and developed a comprehensive and compelling HFE/UE report for inclusion in Insmed's NDA submission. Our support helped enable Insmed to submit its NDA on time and with the data required by FDA to assess use-safety of the Lamira® nebulizer system. ARIKAYCE was approved by FDA in September 2018.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.