Device description
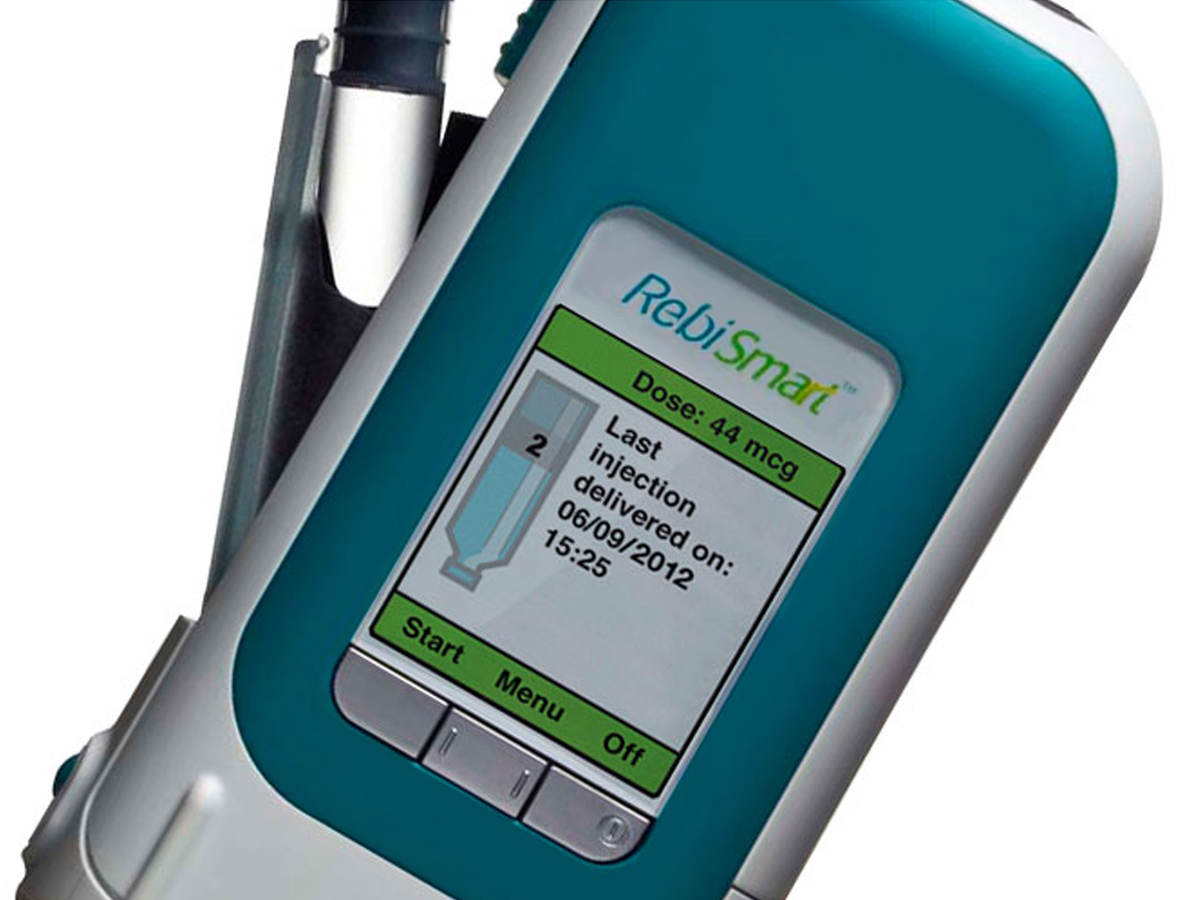
RebiSmart is an electronic autoinjector designed to administer a preset dose of Rebif (interferon beta-1a) for treatment of relapsing multiple sclerosis (RMS). The cartridge-based autoinjector has a large, full-color embedded software user interface that enables patients to receive injection reminders and review their injection history. Via the same user interface, healthcare providers can tailor various injection comfort settings, including the speed, depth, and time, to optimize patient convenience.
Challenge
Merck KGaA initially sought to evaluate the autoinjector and instructions' usability and use-safety, and to identify opportunities to improve the design-in-progress. And, more generally, Merck KGaA sought to apply human factors engineering to ensure the autoinjector enabled safer and more effective use, and to meet regulators' expectations.
Solution
We conducted an expert review (i.e., design critique) of RebiSmart's instructions for use and identified opportunities to improve the document's usability and clarity, considering text and graphics, as well as overall organization and information presentation. Then, Merck KGaA engaged us to plan, conduct, and report the results of HF pre-validation and validation tests of the device and its instructions. We also provided regulatory consulting support, helping Merck KGaA ensure compliance with applicable HFE standards, guidance documents, and notified bodies' expectations, and participating in meetings with regulators to discuss human factors.
Impact
Through the design critique and usability testing, we provided our input, and shared valuable insights from many RMS patients, caregivers, and healthcare providers, to help improve RebiSmart's usability (e.g., improved injection convenience) and use-safety. Coupled with regulatory consulting support, we helped Merck KGaA optimize RebiSmart and receive approval to market the device in Europe.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.
Related HFR&D services
- Expert Early-Stage Consulting for Medical Device Development
- Enhance Medical Device Development with Comprehensive User Research
- Comprehensive Human Factors Analysis for Medical Device Development
- Optimizing User Experience for Medical Devices
- Medical Device Evaluation and Usability Testing
- Human Factors Engineering Training and Consulting